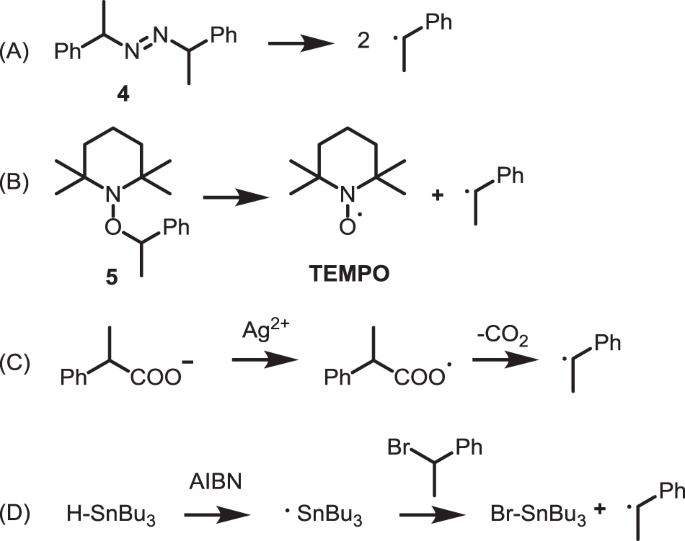
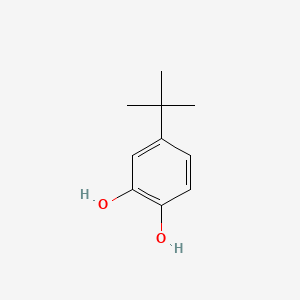
Mechanistic study of electrochemical oxidation of 4-tert-butylcatechol: A facile electrochemical method for the synthesis of new trimer of 4-tert- butylcatechol - ScienceDirect

Investigation of the electro-methoxylation reaction: Part 1. Electrochemical study of 4-tert-butylcatechol and 3,4-dihydroxybenzaldehyde in methanol - ScienceDirect

Peroxy Radical Activated Addition of tert-Butylcatechol to 2,6-Di-tert-butyl-7-Substituted Quinone Methide Polymerization Retarders | Organic Process Research & Development

Electrochemical study of 4-tert-butylcatechol in the presence of 1,3-dimethylbarbituric acid and 1,3-diethyl-2-thiobarbituric acid. Application to the electro-organic synthesis of new corresponding spiropyrimidine derivatives - ScienceDirect
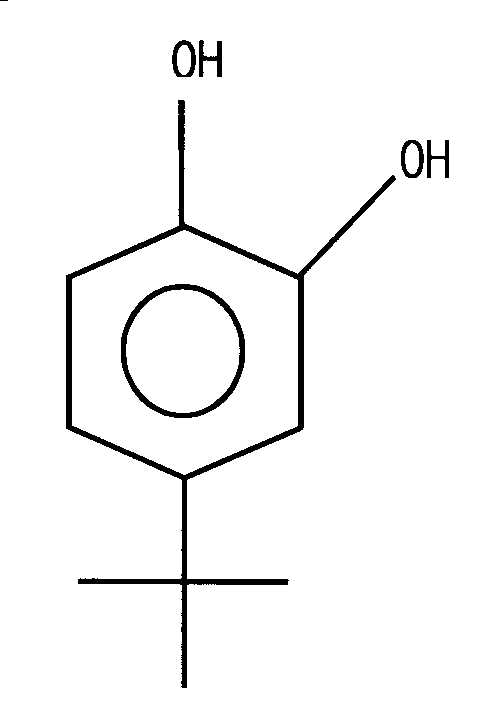
KR100676996B1 - Polymerization inhibitor for 1,3-butadiene and a method of inhibiting polymerization of 1,3-butadiene by imputing thereof - Google Patents
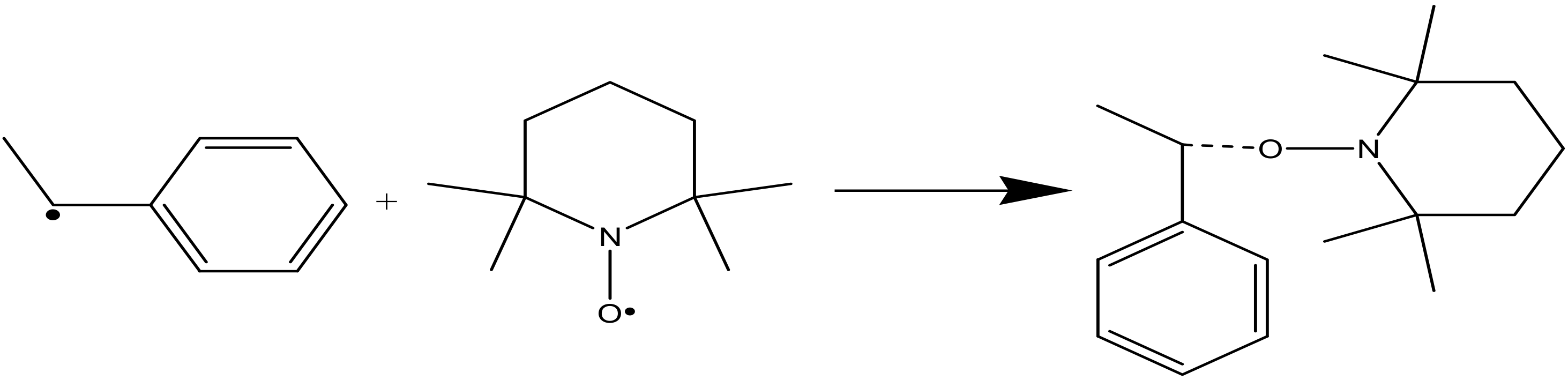
Processes | Free Full-Text | A Theoretical and Experimental Study for Screening Inhibitors for Styrene Polymerization

A non-covalent complex based on catechol–benzoxazole moieties: electrochemical synthesis and characterization - RSC Advances (RSC Publishing) DOI:10.1039/C4RA02340D
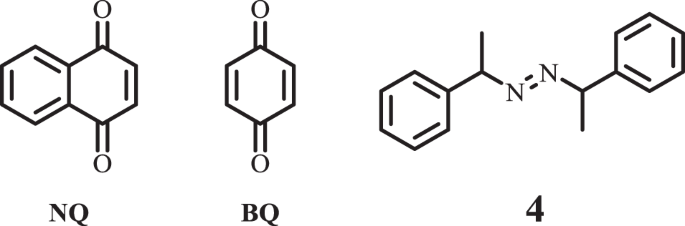
Polymerization inhibition mechanism of 1,4-naphthoquinone by experimentation and DFT calculations | Polymer Journal
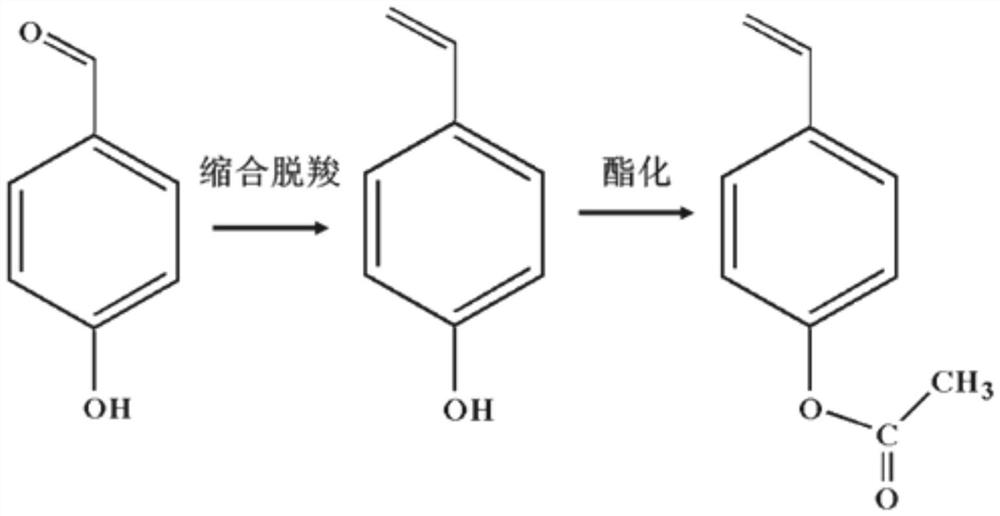
Method for preparing p-acetoxystyrene by one-pot method - Eureka | Patsnap develop intelligence library