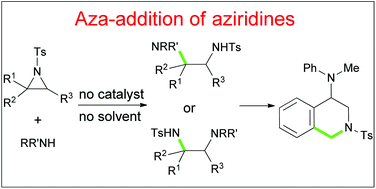
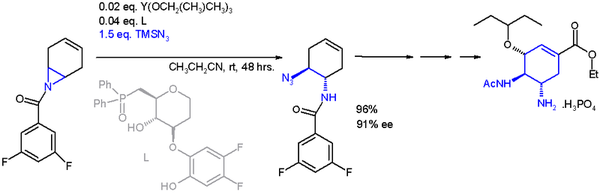
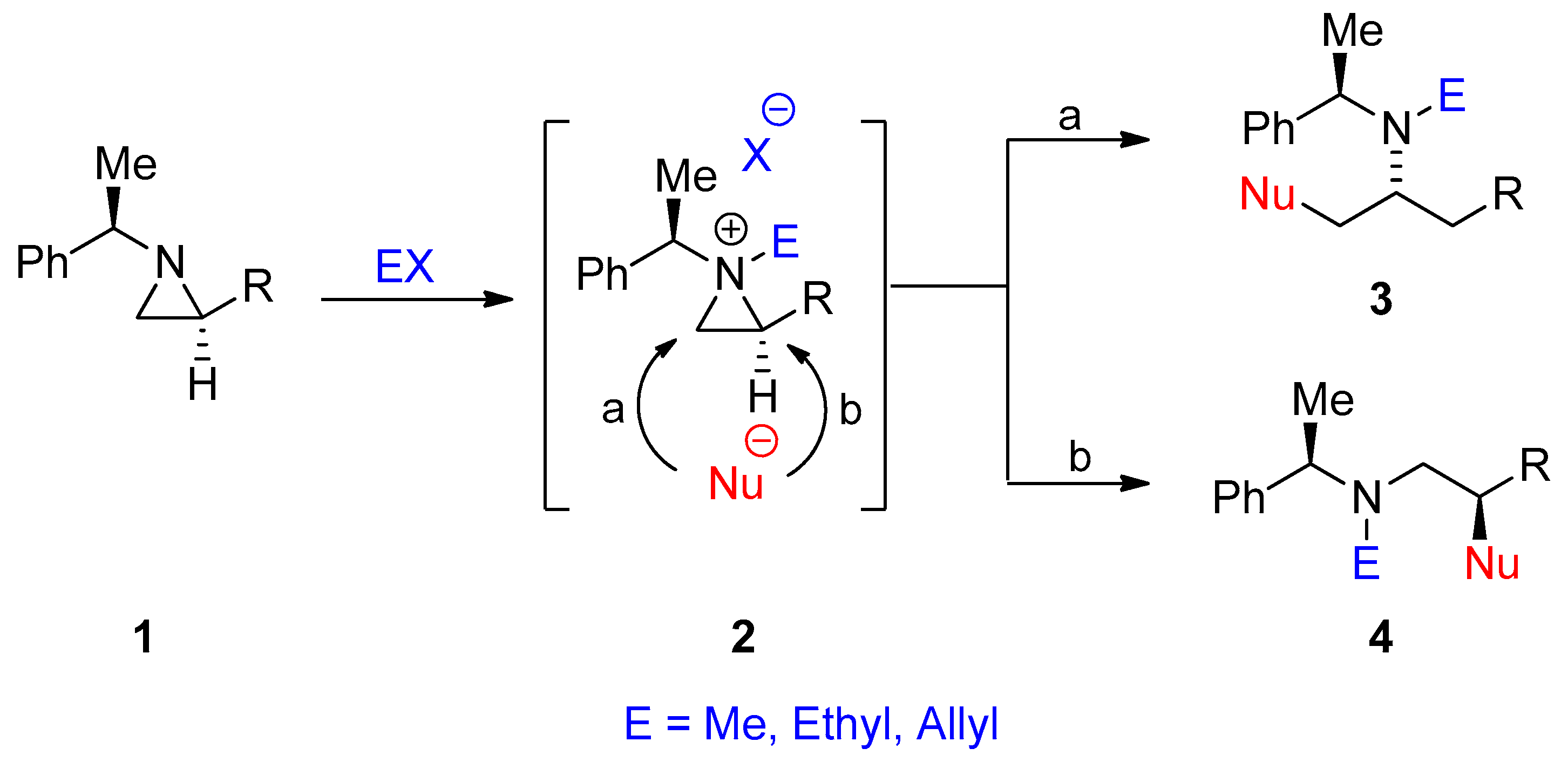
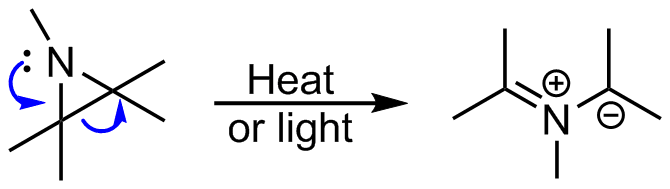
Highly regioselective ring-opening of aziridines with arenesulfinates on water: a facile access to β-amino/vinyl sulfones - ScienceDirect
![N-methylative aziridine ring opening and the synthesis of (S)-3-methylamino-3-[(R)-pyrrolidin-3-yl]propanenitrile - ScienceDirect N-methylative aziridine ring opening and the synthesis of (S)-3-methylamino-3-[(R)-pyrrolidin-3-yl]propanenitrile - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S0040402017308839-fx1.jpg)
N-methylative aziridine ring opening and the synthesis of (S)-3-methylamino-3-[(R)-pyrrolidin-3-yl]propanenitrile - ScienceDirect
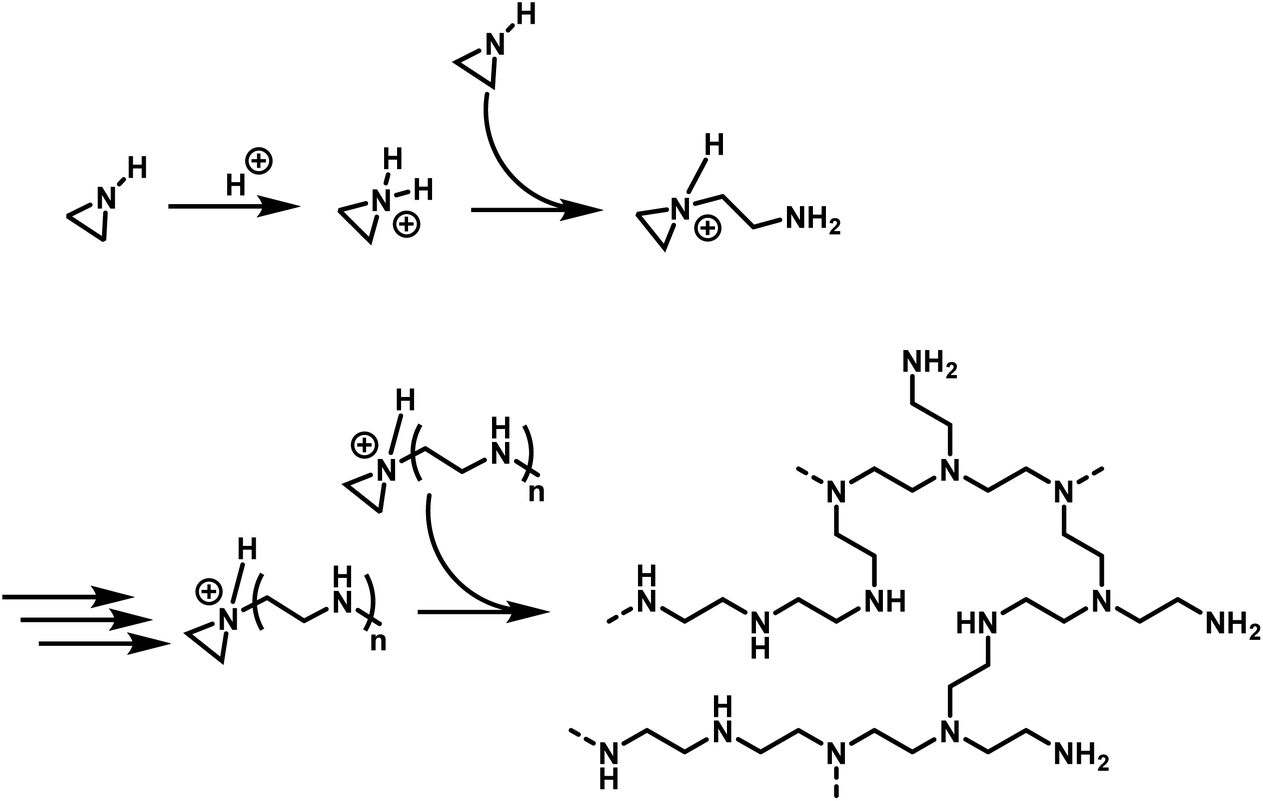
Aziridines and azetidines: building blocks for polyamines by anionic and cationic ring-opening polymerization - Polymer Chemistry (RSC Publishing) DOI:10.1039/C9PY00278B

Regioselective Synthesis of N-β-Hydroxyethylaziridines by the Ring-Opening Reaction of Epoxides with Aziridine Generated in Situ
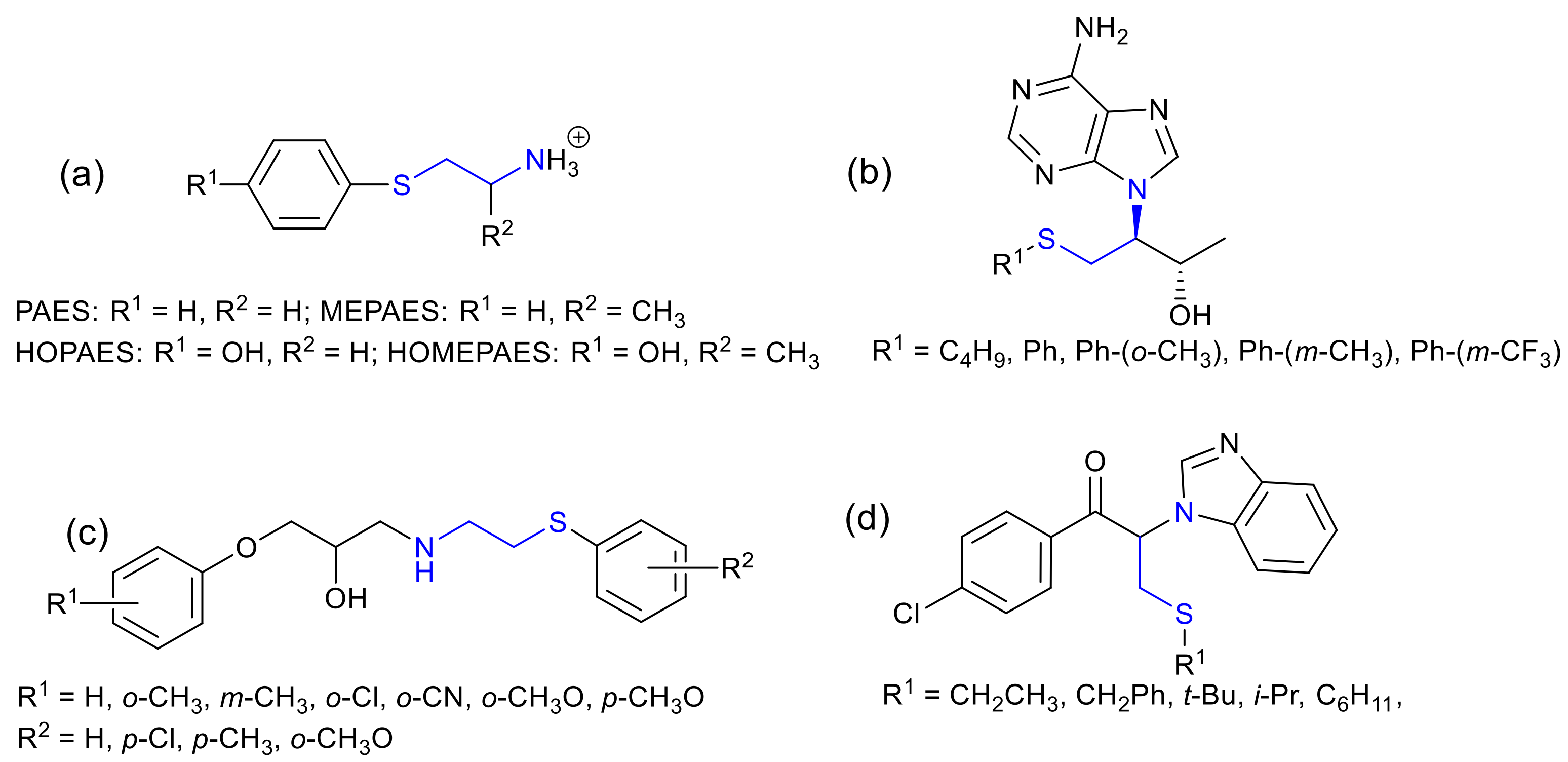
Molecules | Free Full-Text | Aziridine Ring Opening as Regio- and Stereoselective Access to C-Glycosyl-Aminoethyl Sulfide Derivatives

Catalyst‐Free Regio‐ and Stereospecific Synthesis of β‐Sulfonamido Dithiocarbamates: Efficient Ring‐Opening Reactions of N‐Tosyl Aziridines by Dialkyldithiocarbamates - Alagiri - 2011 - Chemistry – A European Journal - Wiley Online Library

Modular, One-Pot, Sequential Aziridine Ring Opening-S(N)Ar Strategy to 7-, 10-, and 11-Membered Benzo-Fused Sultams. - Abstract - Europe PMC
![PDF] Recent applications of aziridine ring expansion reactions in heterocyclic synthesis | Semantic Scholar PDF] Recent applications of aziridine ring expansion reactions in heterocyclic synthesis | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/e154a08385bc9b0b26216a1d8f7b25260b67df13/3-Figure1-1.png)
PDF] Recent applications of aziridine ring expansion reactions in heterocyclic synthesis | Semantic Scholar

Regioselective Ring‐Opening Nucleophilic Addition of Aziridines through Photoredox Catalyst - Sun - 2014 - Advanced Synthesis & Catalysis - Wiley Online Library
![PDF] Recent applications of aziridine ring expansion reactions in heterocyclic synthesis | Semantic Scholar PDF] Recent applications of aziridine ring expansion reactions in heterocyclic synthesis | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/e154a08385bc9b0b26216a1d8f7b25260b67df13/10-Figure2-1.png)
PDF] Recent applications of aziridine ring expansion reactions in heterocyclic synthesis | Semantic Scholar

Enantioselective fluoride ring opening of aziridines enabled by cooperative Lewis acid catalysis - ScienceDirect
Scheme 3. Regioselective ring-opening reactions of the [3.1.0] bicyclic... | Download Scientific Diagram
![Intermolecular [3+3] ring expansion of aziridines to dehydropiperi-dines through the intermediacy of aziridinium ylides | Nature Communications Intermolecular [3+3] ring expansion of aziridines to dehydropiperi-dines through the intermediacy of aziridinium ylides | Nature Communications](https://media.springernature.com/m685/springer-static/image/art%3A10.1038%2Fs41467-020-15134-x/MediaObjects/41467_2020_15134_Fig1_HTML.png)
Intermolecular [3+3] ring expansion of aziridines to dehydropiperi-dines through the intermediacy of aziridinium ylides | Nature Communications
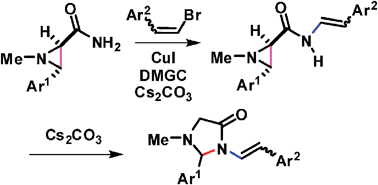
Unprecedented carbon–carbon bond cleavage in nucleophilic aziridine ring opening reaction, efficient ring transformation of aziridines to imidazolidin-4-ones - Chemical Communications (RSC Publishing)
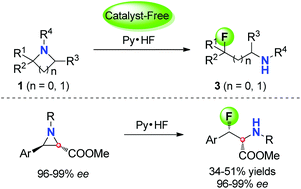
Highly efficient regio-selective ring-opening nucleophilic fluorination of aziridines and azetidines: access to β- or γ-fluorinated amino acid derivatives - Organic & Biomolecular Chemistry (RSC Publishing)