
Regiospecific synthesis of 6-halouridine derivatives: An effective method for coupling sterically hindered pyrimidine bases to ribose - ScienceDirect

N,O-Bis(trimethylsilyl)trifluoroacetamide with trimethylchlorosilane with 1 trimethylchlorosilane, for GC derivatization, LiChropur 25561-30-2

N,O-Bis(trimethylsilyl)acetamide/N-hydroxysuccinimide ester (BSA/NHS) as coupling agents for dipeptide synthesis - ScienceDirect

Chiral Quaternary Ammonium Aryloxide/N,O‐Bis(trimethyl‐ silyl)acetamide Combination as Efficient Organocatalytic System for the Direct Vinylogous Aldol Reaction of (5H)‐Furan‐2‐one Derivatives - Claraz - 2013 - Advanced Synthesis & Catalysis ...
N,O-Bis(trimethylsilyl) acetamide (BSA) | Reagents for Silylation | Derivatisation Reagents for GC | Gas Chromatography (GC) | Chromatography | Applications | Carl Roth - Austria
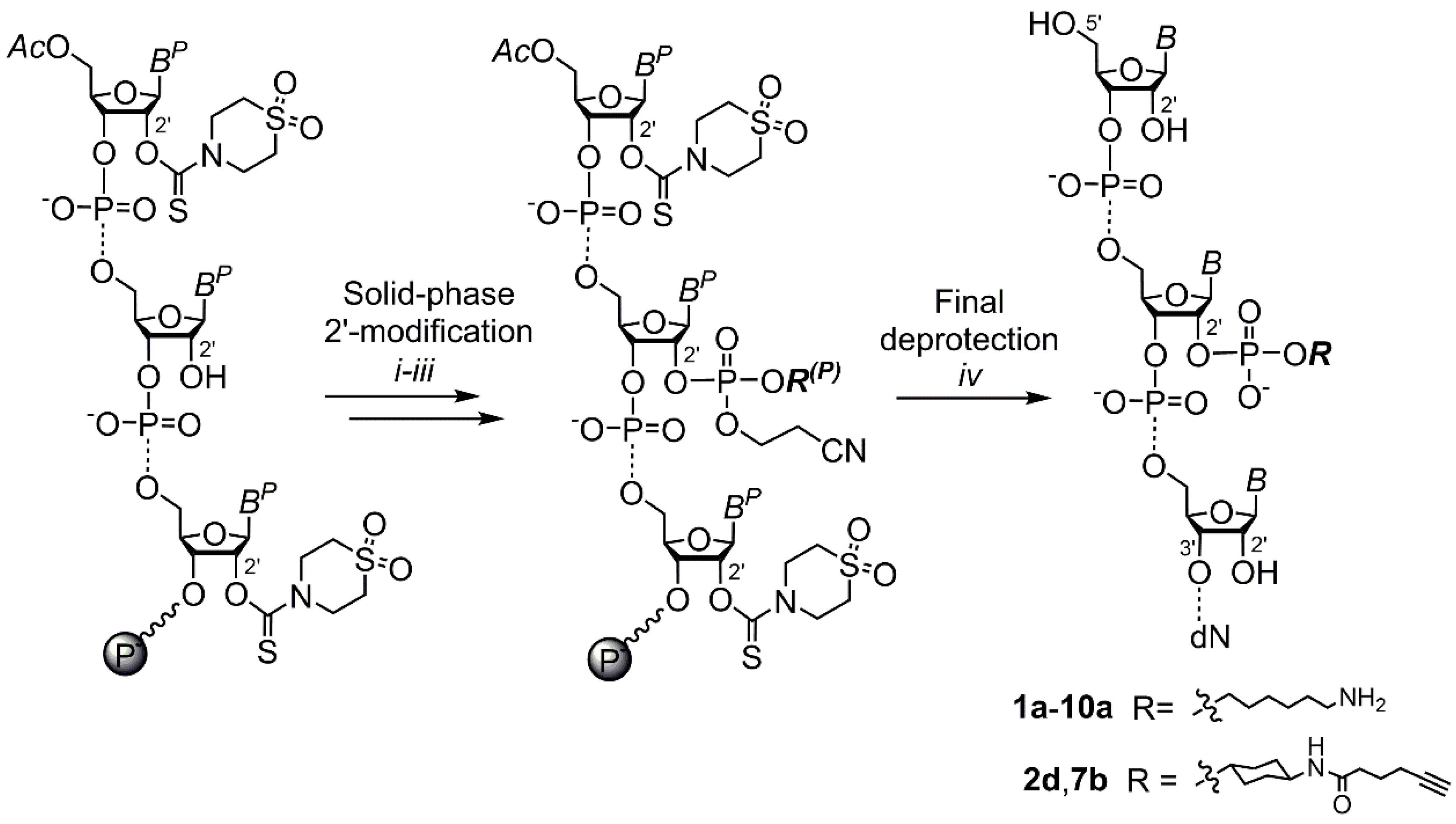
IJMS | Free Full-Text | Postsynthetic On-Column 2′ Functionalization of RNA by Convenient Versatile Method | HTML

Recent extensions of the Morita–Baylis–Hillman reaction - Chemical Communications (RSC Publishing) DOI:10.1039/B909405A

Synthesis of 4‐Quinolones: N,O‐Bis(trimethylsilyl)acetamide‐Mediated Cyclization with Cleavage of Aromatic C–O Bond - Píša - 2016 - European Journal of Organic Chemistry - Wiley Online Library
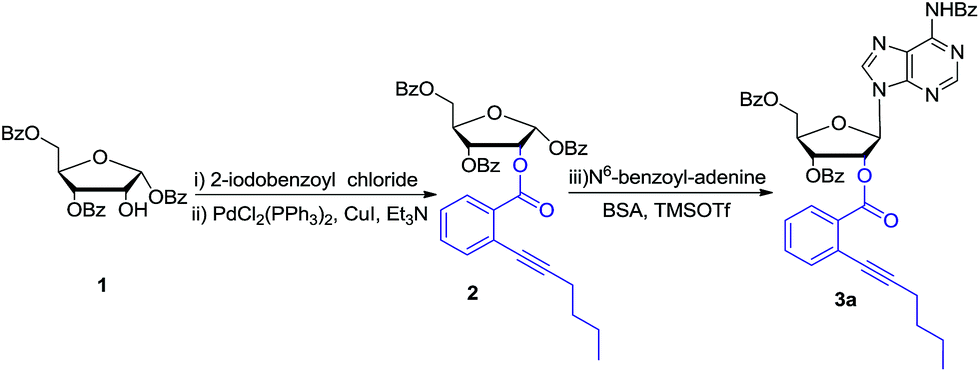
Stereoselective synthesis of 2′-modified nucleosides by using ortho -alkynyl benzoate as a gold( i )-catalyzed removable neighboring participation gro ... - RSC Advances (RSC Publishing) DOI:10.1039/C6RA27790J
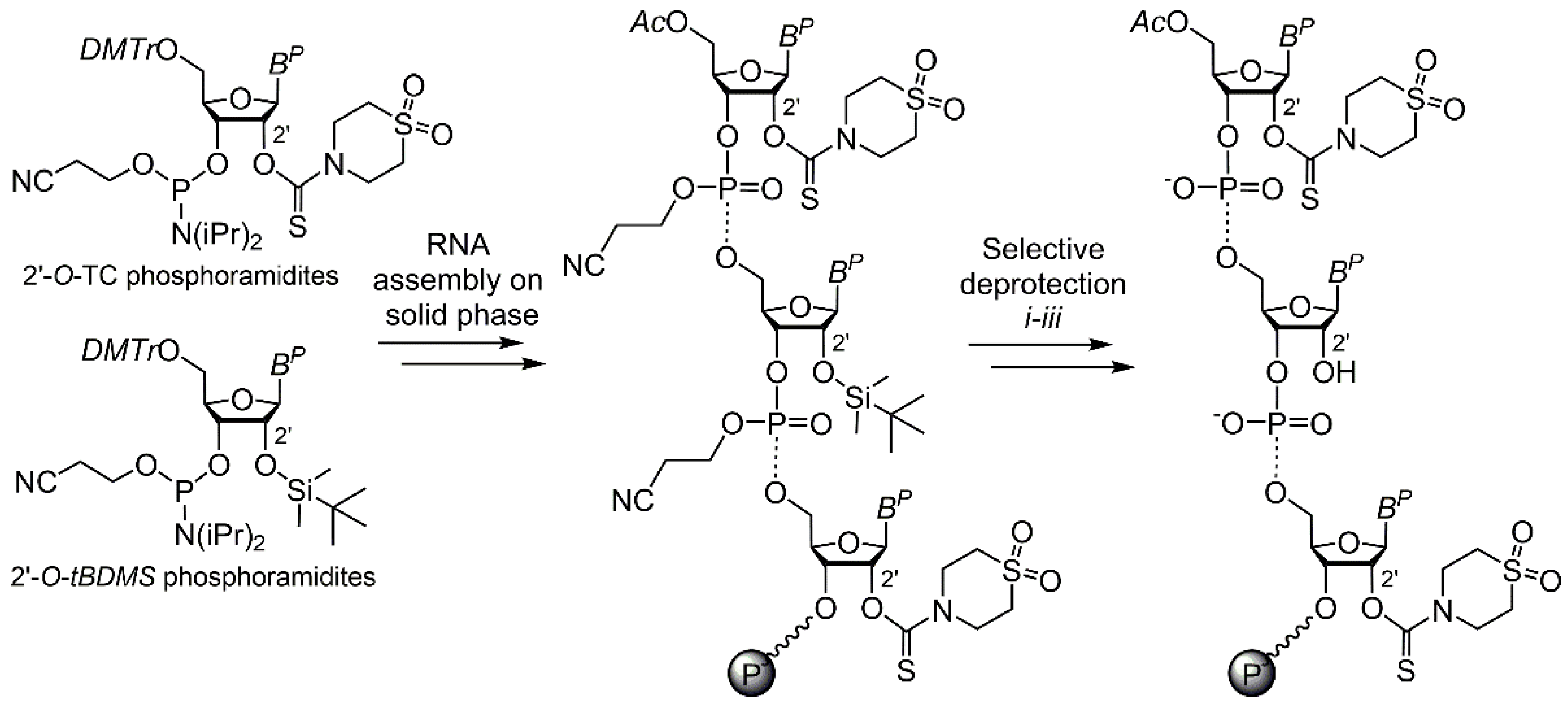
IJMS | Free Full-Text | Postsynthetic On-Column 2′ Functionalization of RNA by Convenient Versatile Method | HTML

Two 3'-O-β-glucosylated nucleoside fluorometabolites related to nucleocidin in Streptomyces calvus. - Abstract - Europe PMC

Synthesis of 4‐Quinolones: N,O‐Bis(trimethylsilyl)acetamide‐Mediated Cyclization with Cleavage of Aromatic C–O Bond - Píša - 2016 - European Journal of Organic Chemistry - Wiley Online Library

Synthesis of 4‐Quinolones: N,O‐Bis(trimethylsilyl)acetamide‐Mediated Cyclization with Cleavage of Aromatic C–O Bond - Píša - 2016 - European Journal of Organic Chemistry - Wiley Online Library

Two competitive routes and the mechanism of trans-silylation of N- trimethylsilyl-N-methylacetamide: Quantum chemical and FTIR study - ScienceDirect



/A693F56648FB503F802585F800754D8B/$file/FB03098_structure.png)
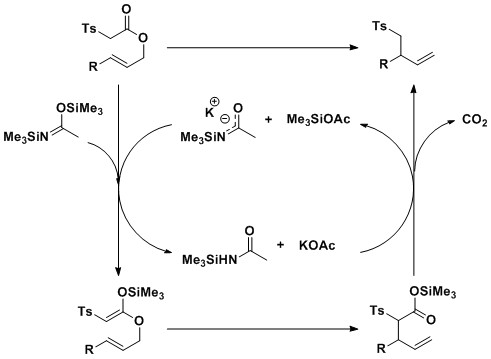

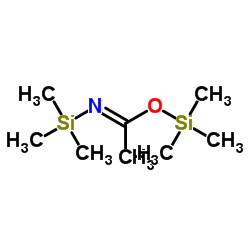
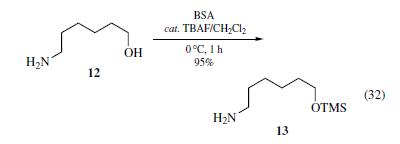



/3A11F417ED478E5E802585F8007D91B8/$file/FB61166_structure.png)