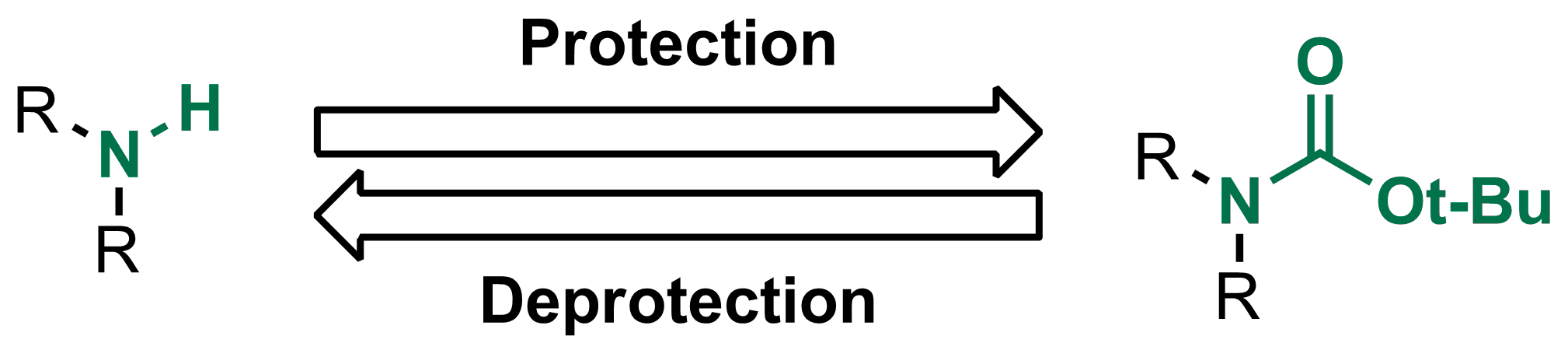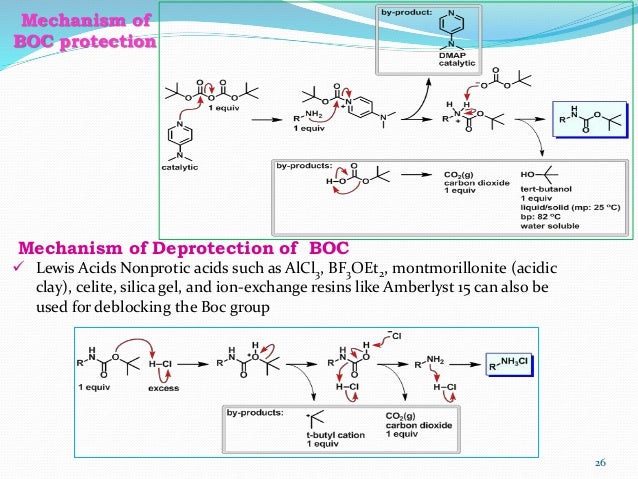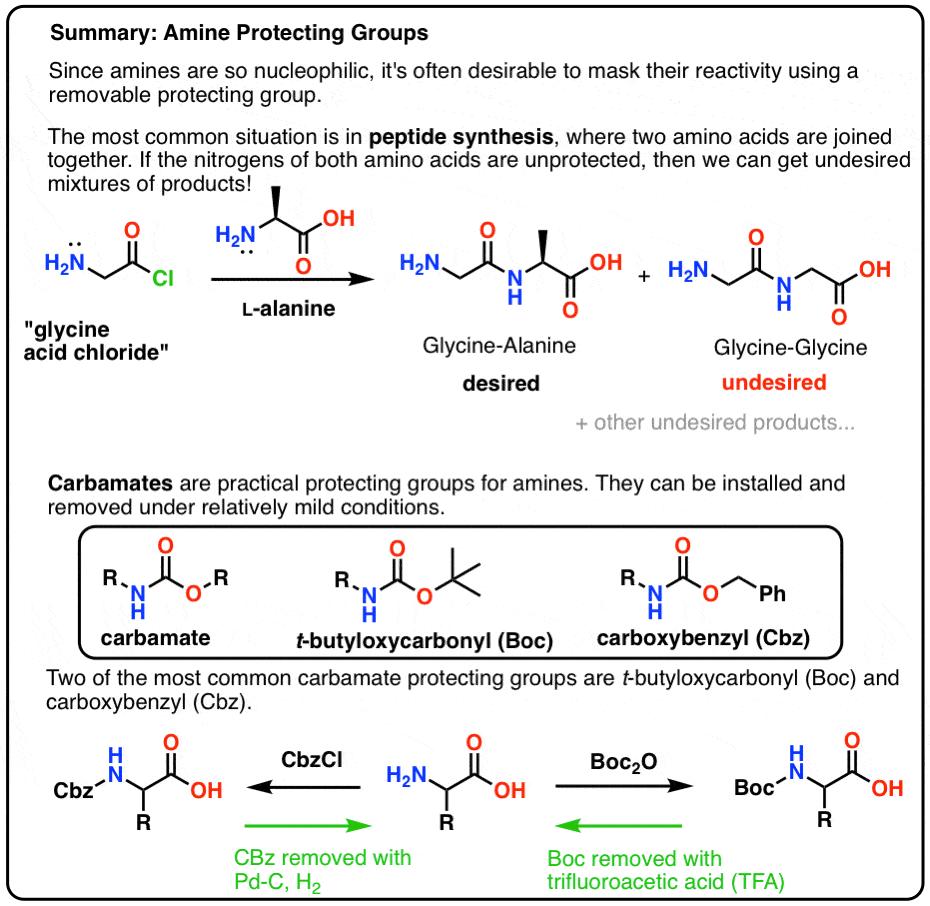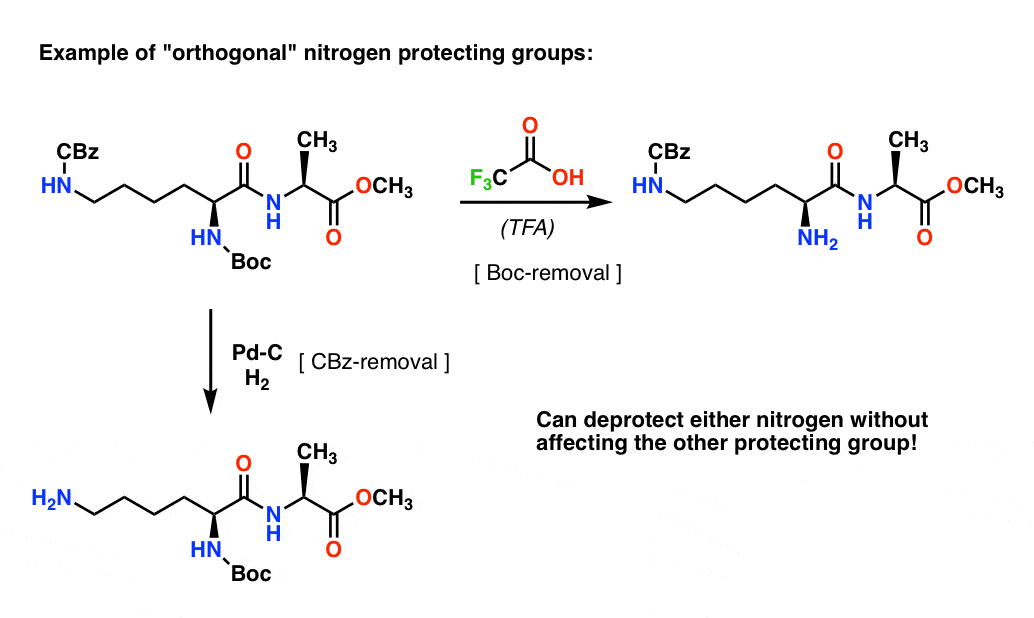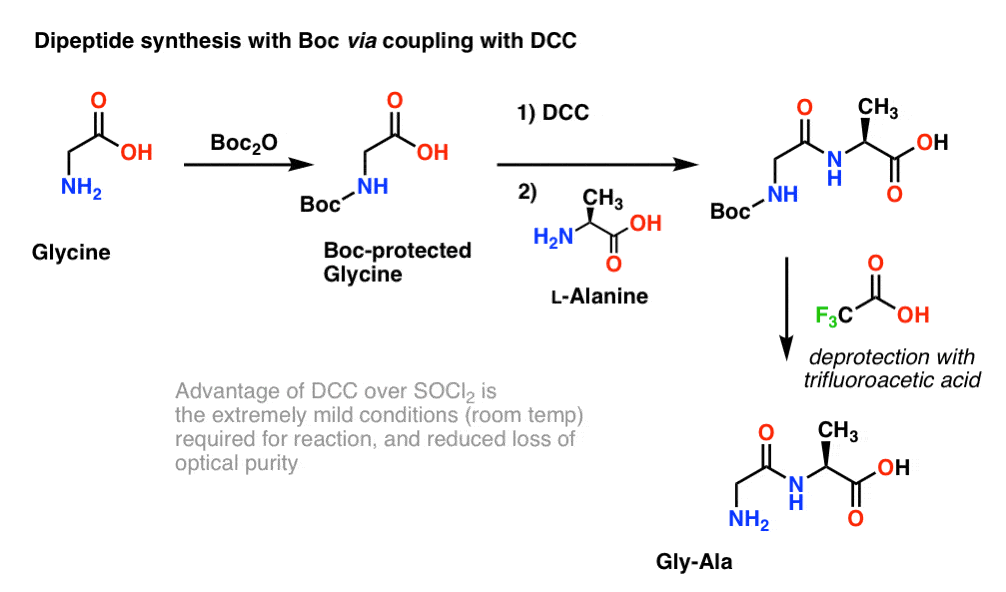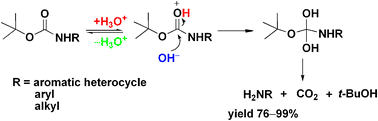
Boiling water-catalyzed neutral and selective N-Boc deprotection - Chemical Communications (RSC Publishing)
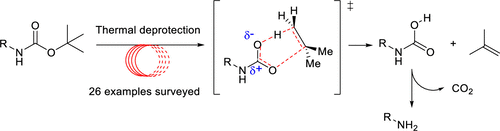
Deprotection of N-Boc Groups under Continuous-Flow High-Temperature Conditions,The Journal of Organic Chemistry - X-MOL

Suppression of Side Reactions During Final Deprotection Employing a Strong Acid in Boc Chemistry: Regeneration of Methionyl Residues from Their Sulfonium Salts | SpringerLink
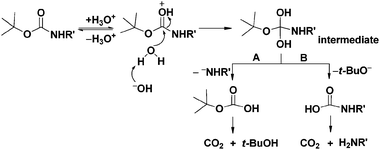
Boiling water-catalyzed neutral and selective N-Boc deprotection - Chemical Communications (RSC Publishing)
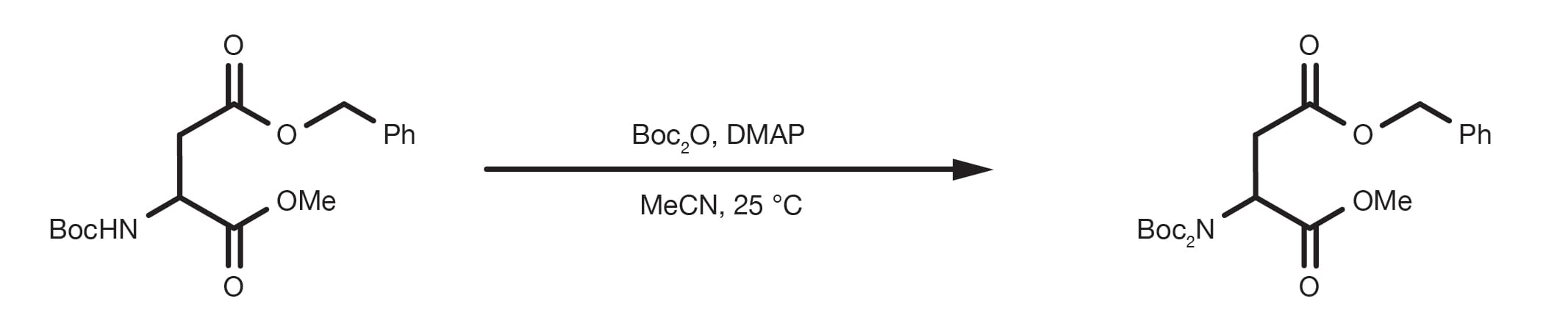
A practical, catalytic and selective deprotection of a Boc group in N,N′-diprotected amines using iron(iii)-catalysis