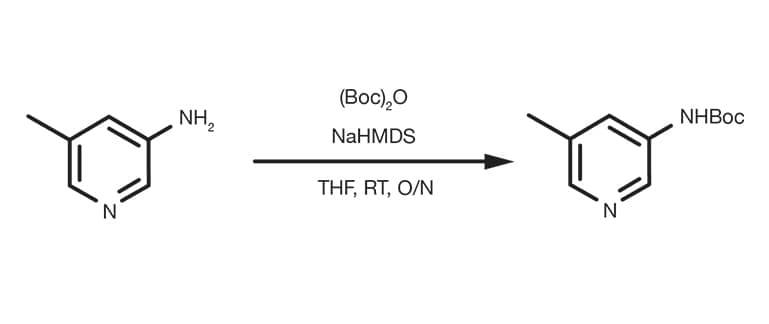
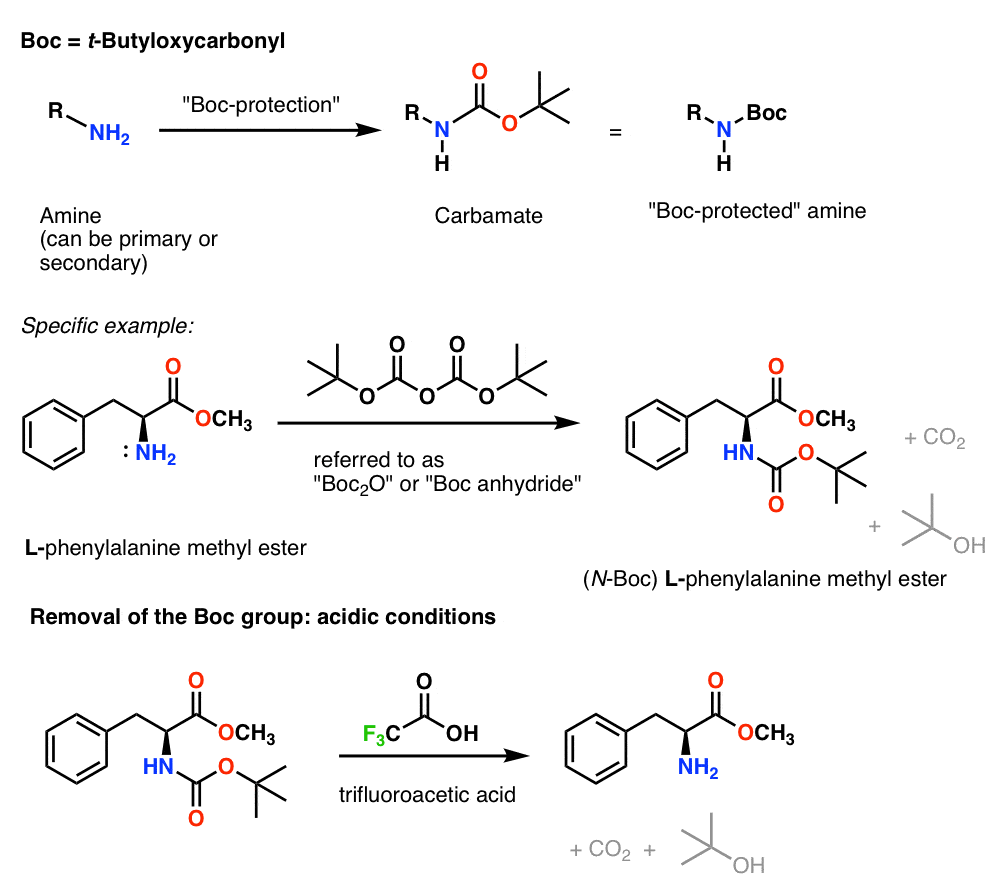
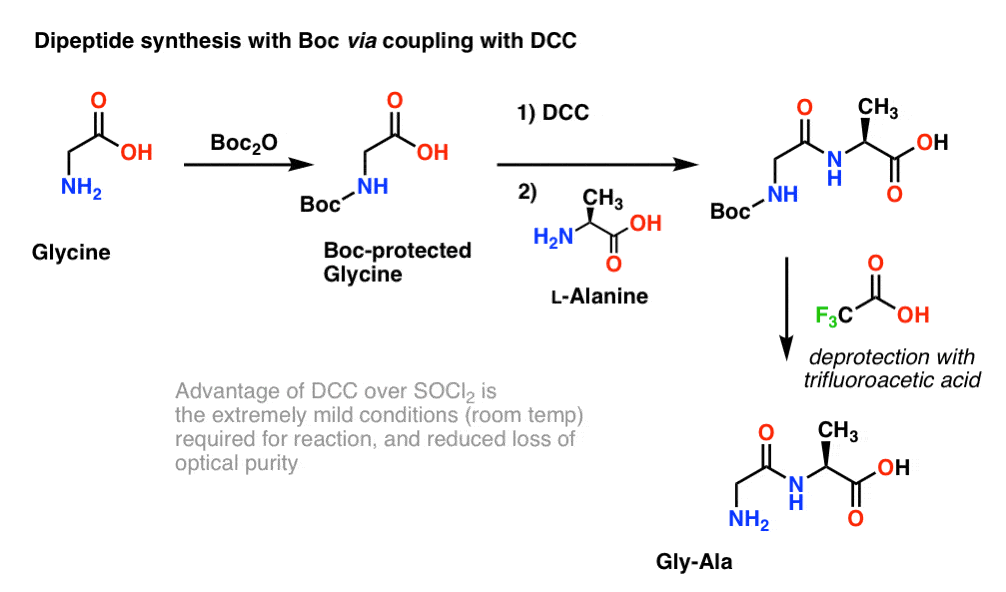
Efficient and expeditious chemoselective BOC protection of amines in catalyst and solvent-free media | SpringerLink
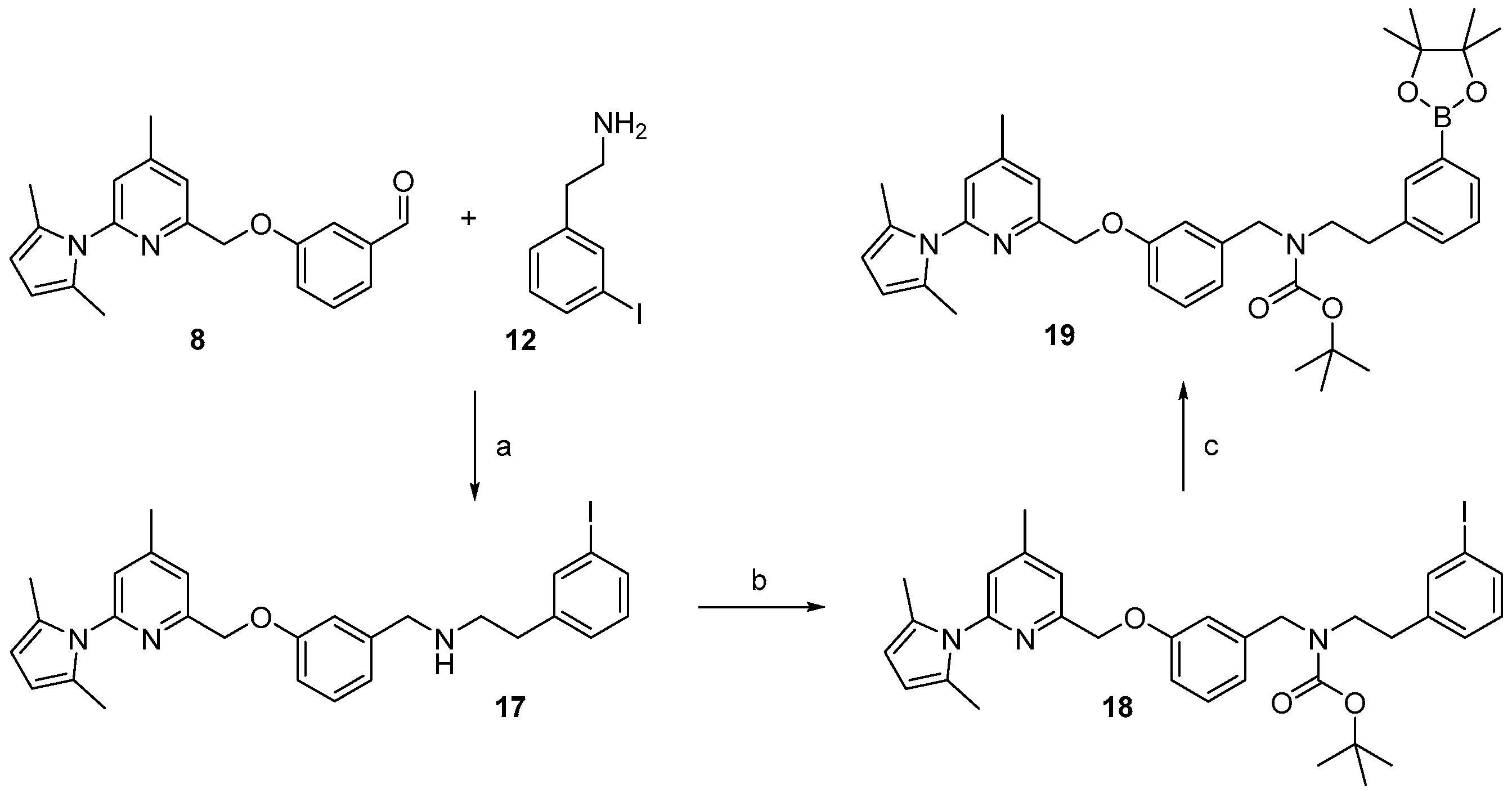
Molecules | Free Full-Text | Synthesis of a Potent Aminopyridine-Based nNOS-Inhibitor by Two Recent No-Carrier-Added 18F-Labelling Methods | HTML

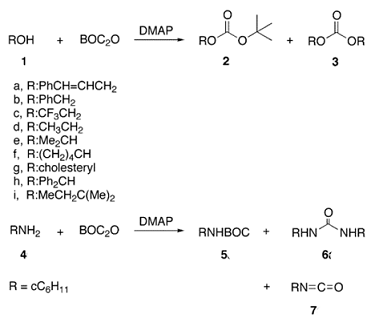
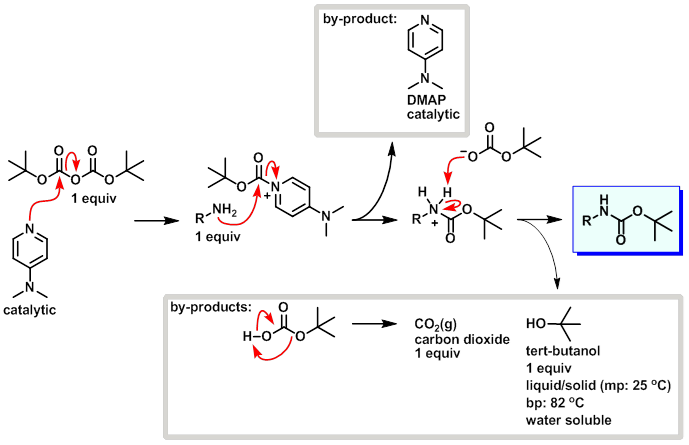
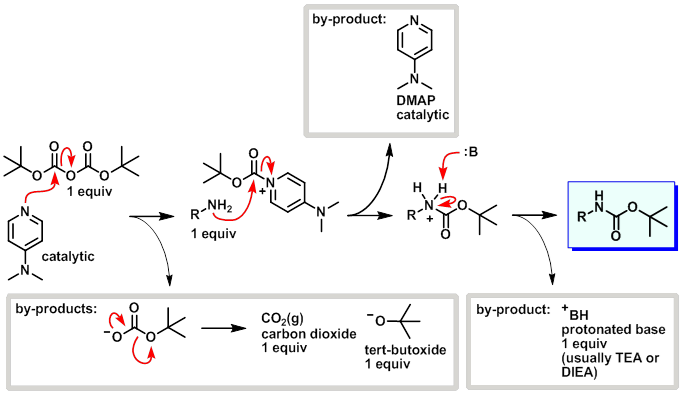
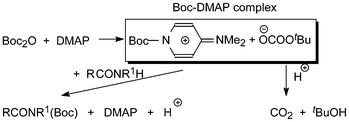
PDF) Di-tert-butyl Dicarbonate and 4-(Dimethylamino)pyridine Revisited. Their Reactions with Amines and Alcohols1
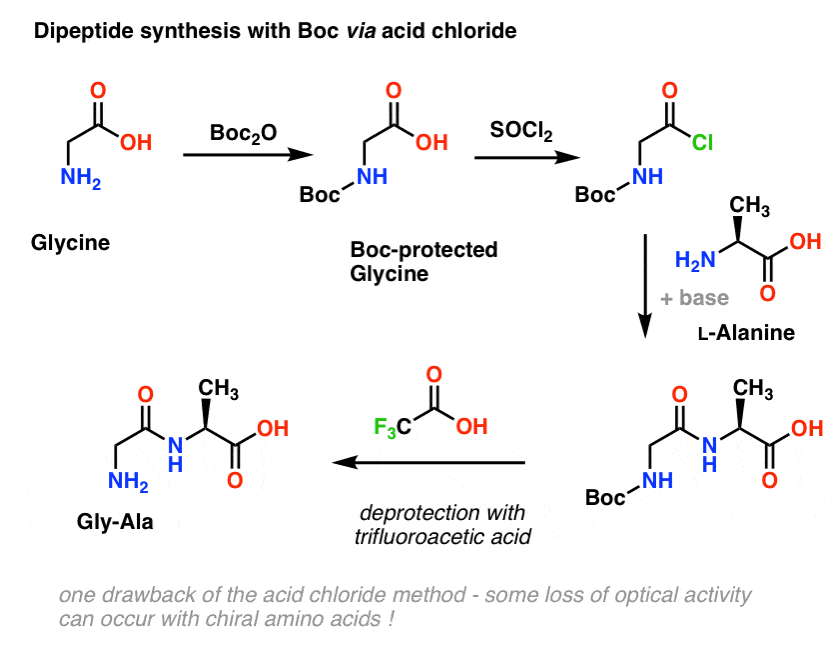
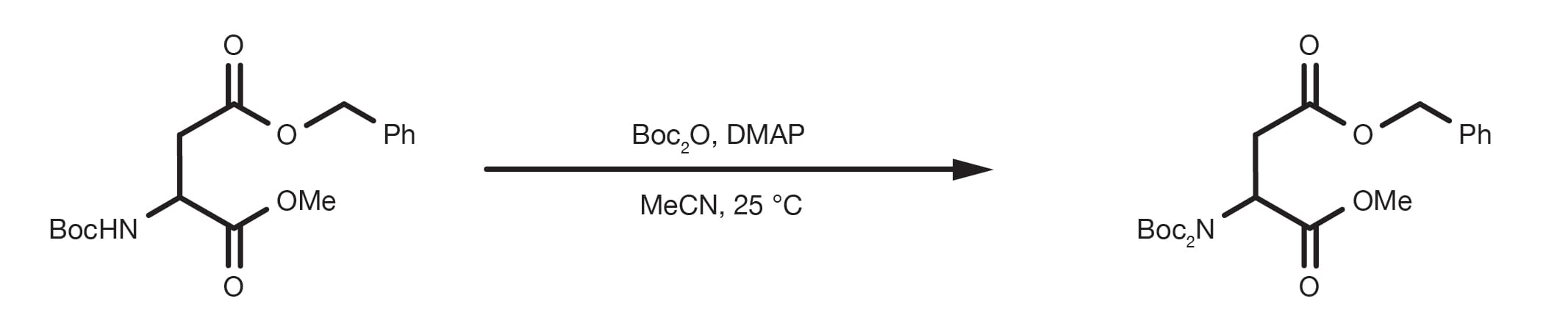
Dual protection of amino functions involving Boc - RSC Advances (RSC Publishing) DOI:10.1039/C3RA42956C

Synthesis of amide derivatives for electron deficient amines and functionalized carboxylic acids using EDC and DMAP and a catalytic amount of HOBt as the coupling reagents - ScienceDirect
Dual protection of amino functions involving Boc - RSC Advances (RSC Publishing) DOI:10.1039/C3RA42956C

An efficient and highly chemoselective N-Boc protection of amines, amino acids, and peptides under heterogeneous conditions | SpringerLink
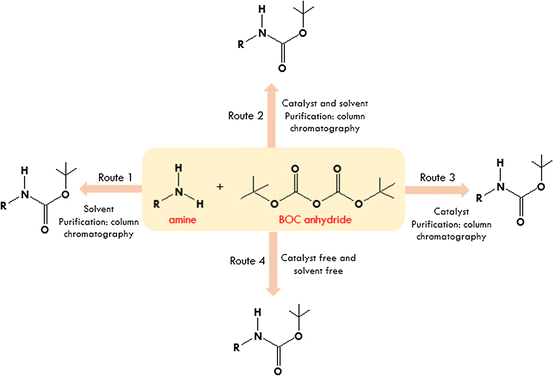
Efficient and expeditious chemoselective BOC protection of amines in catalyst and solvent-free media | SpringerLink