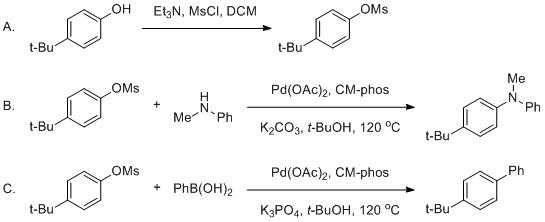
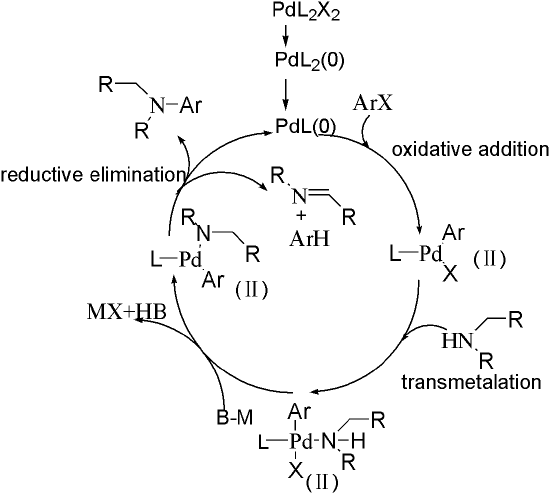
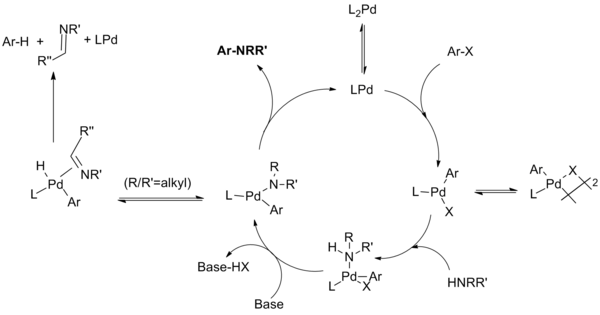
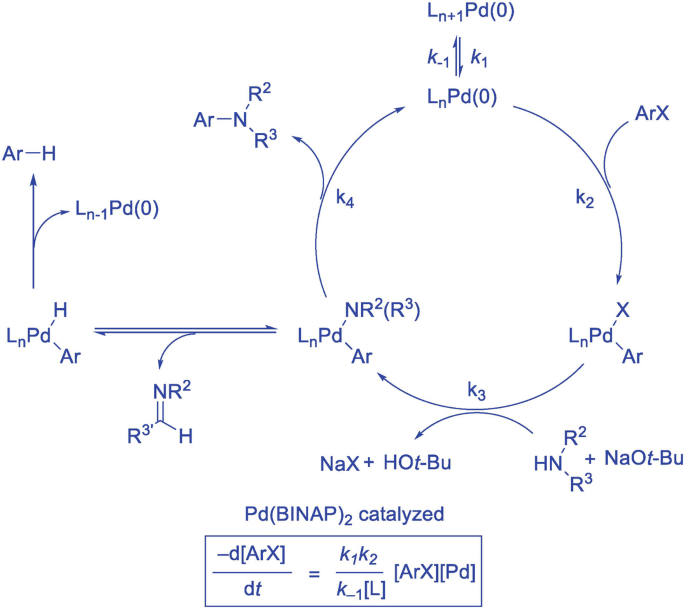
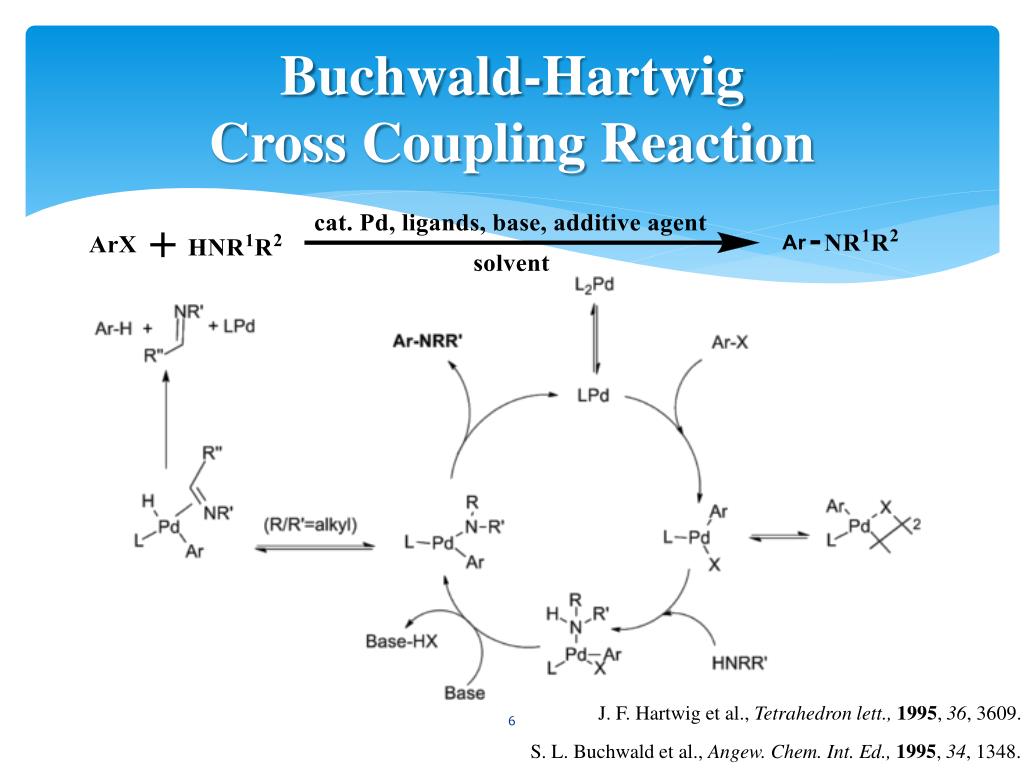
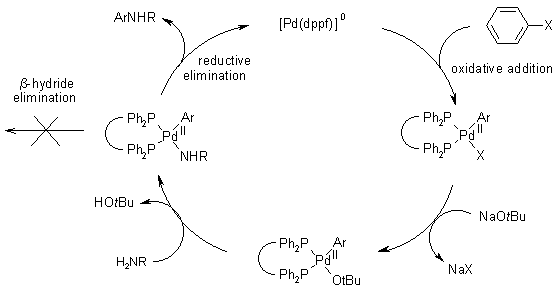
The Buchwald–Hartwig Amination After 25 Years - Dorel - 2019 - Angewandte Chemie International Edition - Wiley Online Library
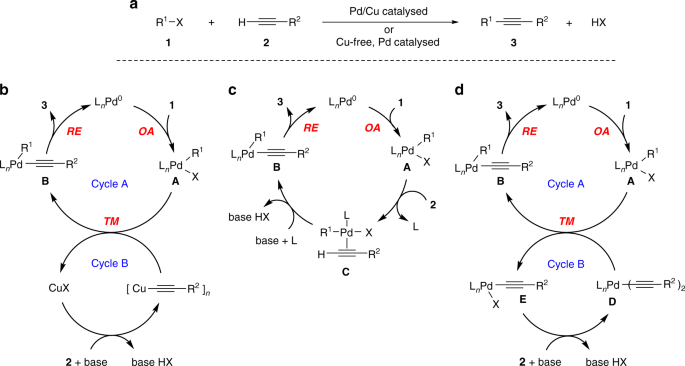
Mechanism of copper-free Sonogashira reaction operates through palladium-palladium transmetallation | Nature Communications

The Buchwald–Hartwig Amination After 25 Years - Dorel - 2019 - Angewandte Chemie International Edition - Wiley Online Library
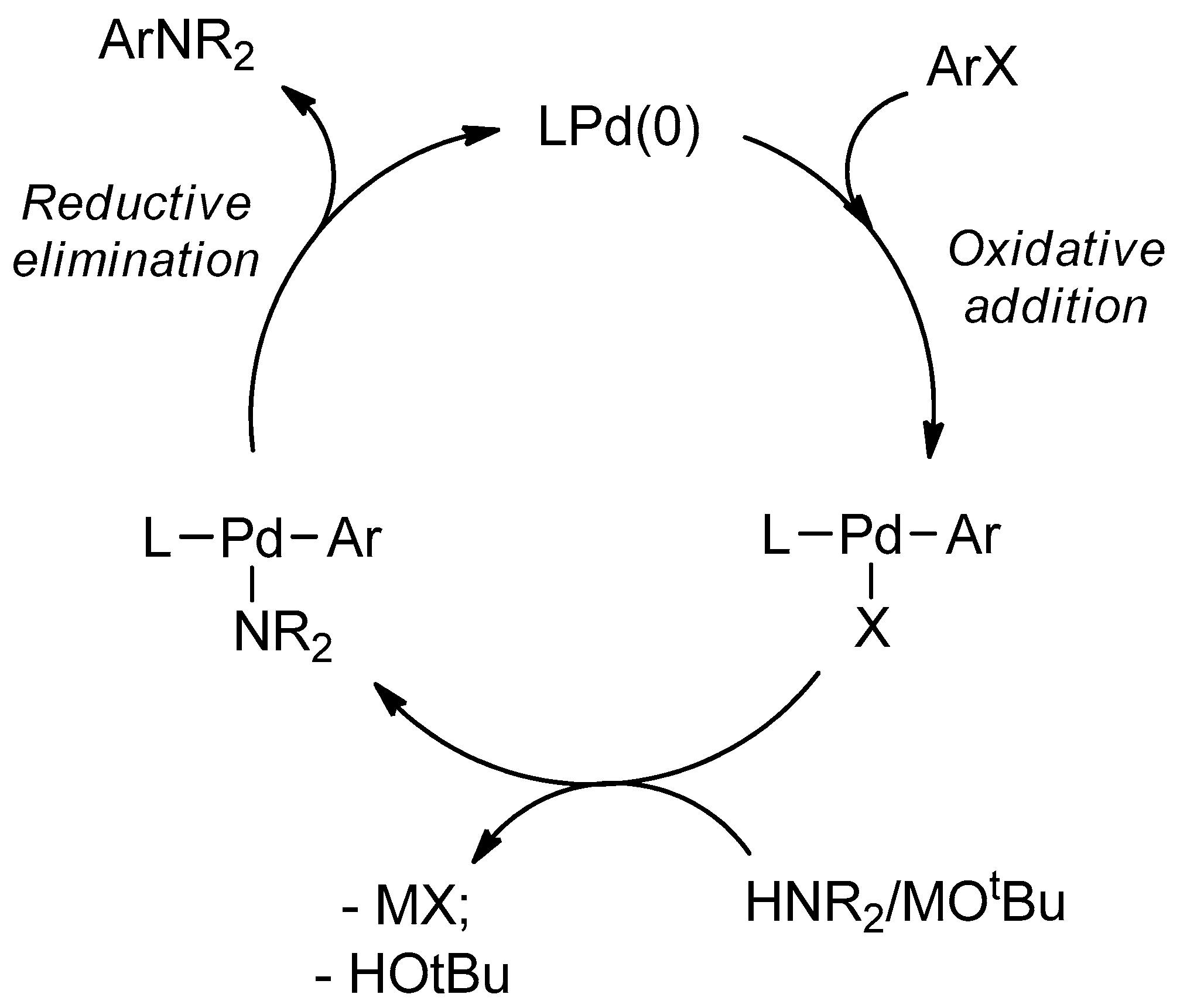
Molecules | Free Full-Text | General Method of Synthesis of 5-(Het)arylamino-1,2,3-triazoles via Buchwald–Hartwig Reaction of 5-Amino- or 5-Halo-1,2,3-triazoles | HTML
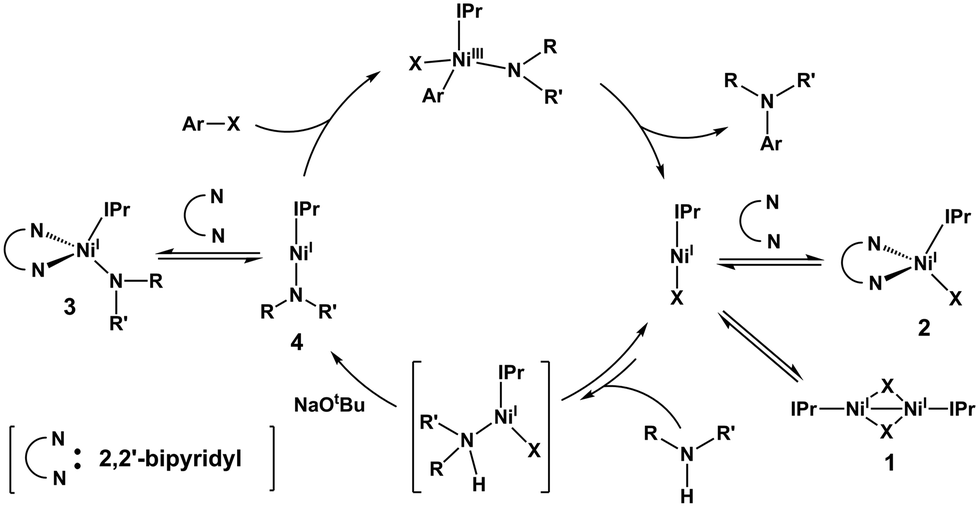
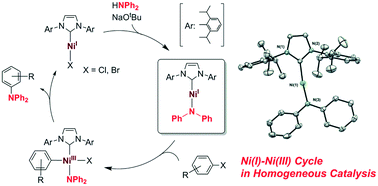
Ni( i )–Ni( iii ) cycle in Buchwald–Hartwig amination of aryl bromide mediated by NHC-ligated Ni( i ) complexes - Catalysis Science & Technology (RSC Publishing) DOI:10.1039/C8CY02427H