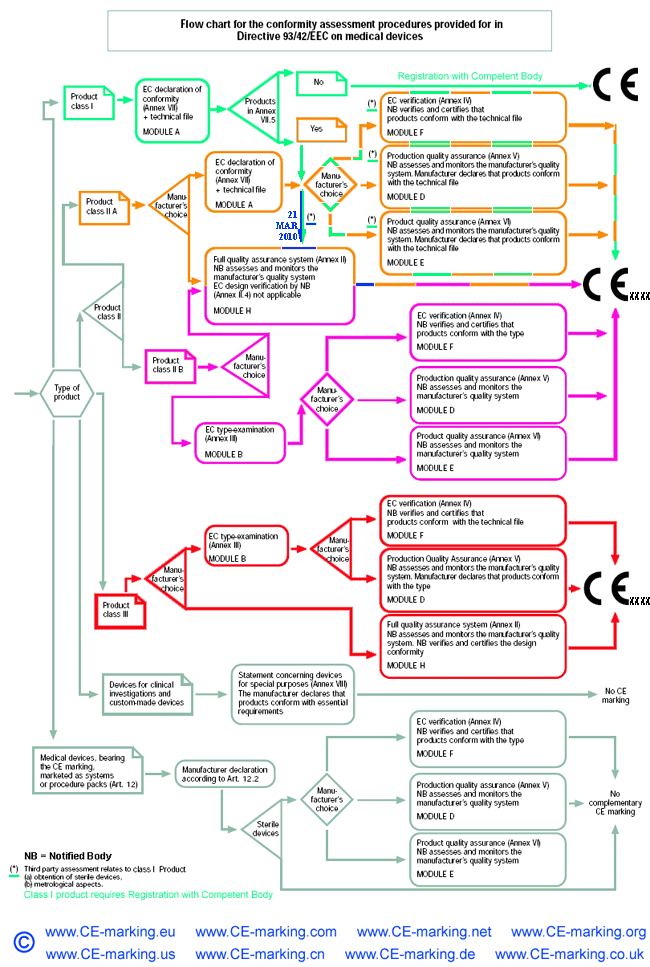
Guide on Class III MDD- Medical Devices CE marking (mark) & European (EU) Authorized Representative service

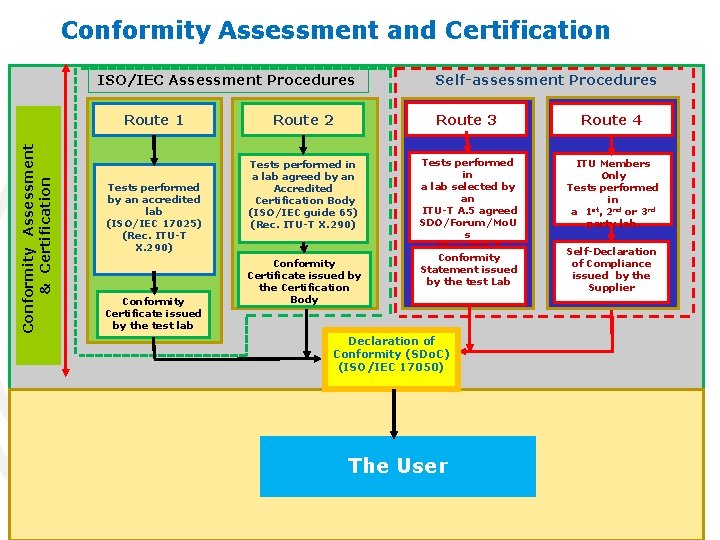
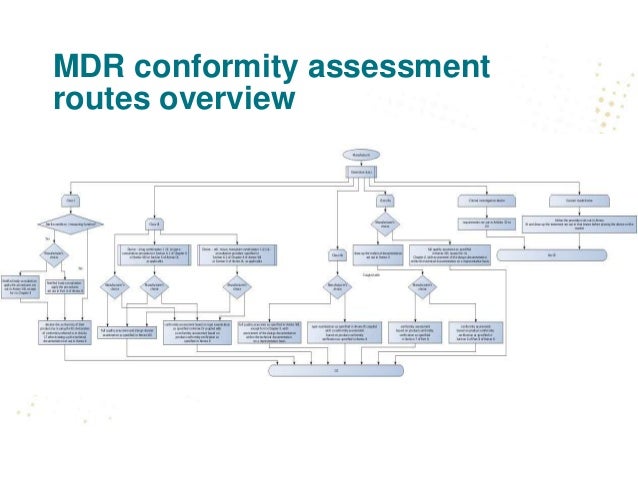
The IVD Regulation – a Simplified Guide to Understanding New Classification and Conformity Assessment Routes
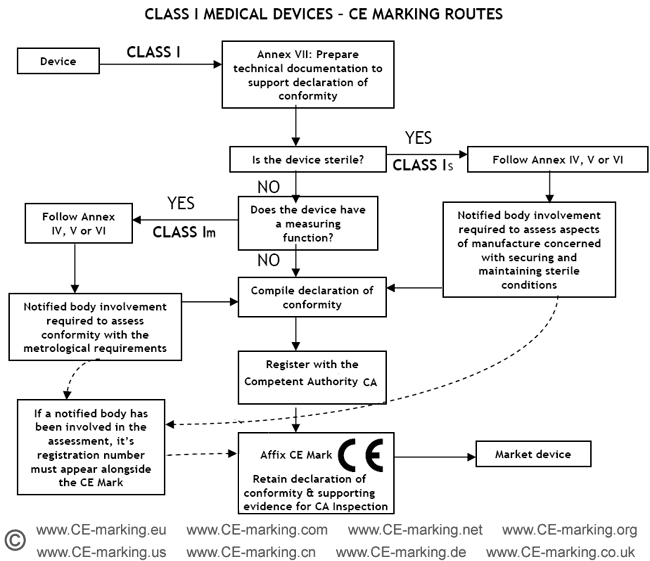
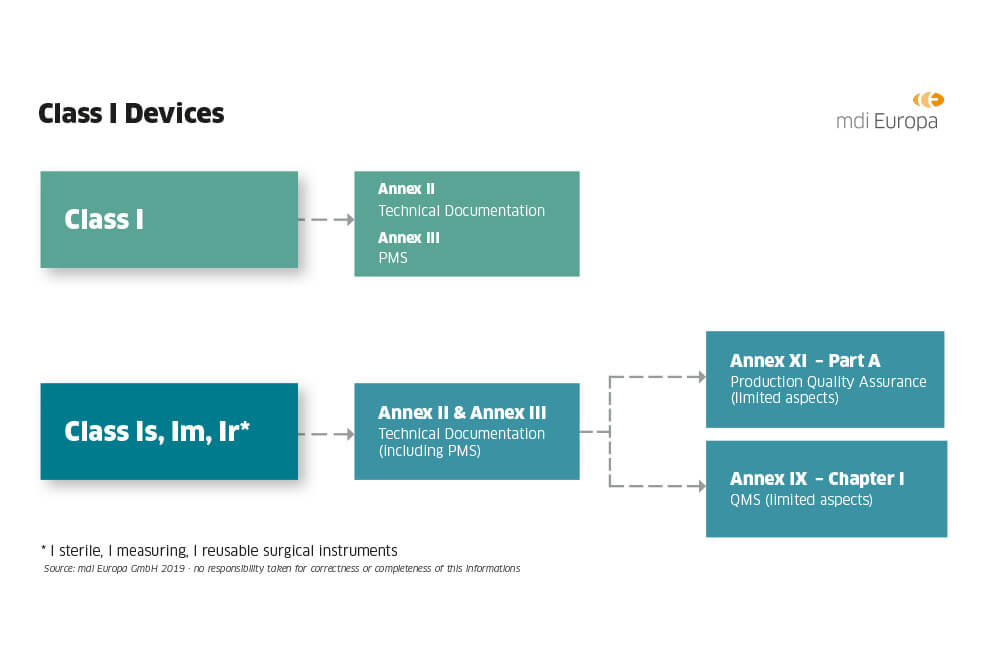
Guide on Class I (Is/Im) MDD- Medical Devices CE marking (mark) & European (EU) Authorized Representative service
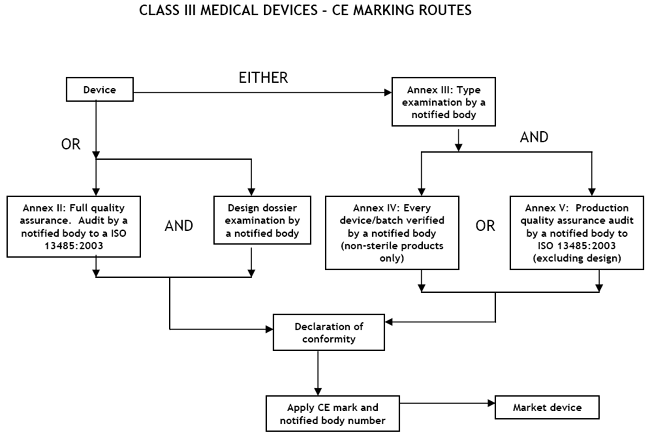
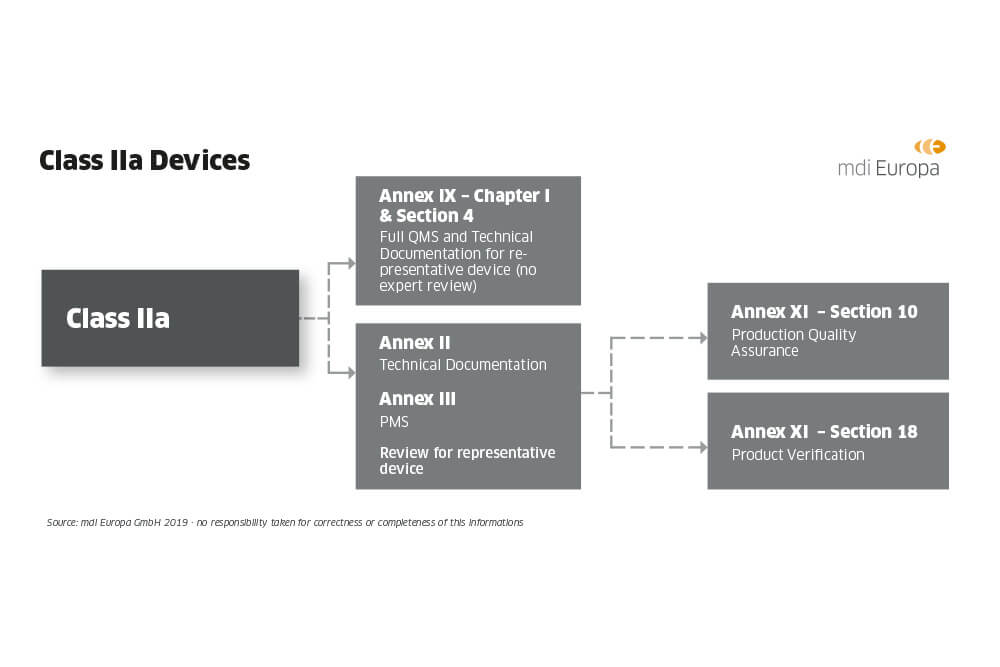
Guide on Class III MDD- Medical Devices CE marking (mark) & European (EU) Authorized Representative service