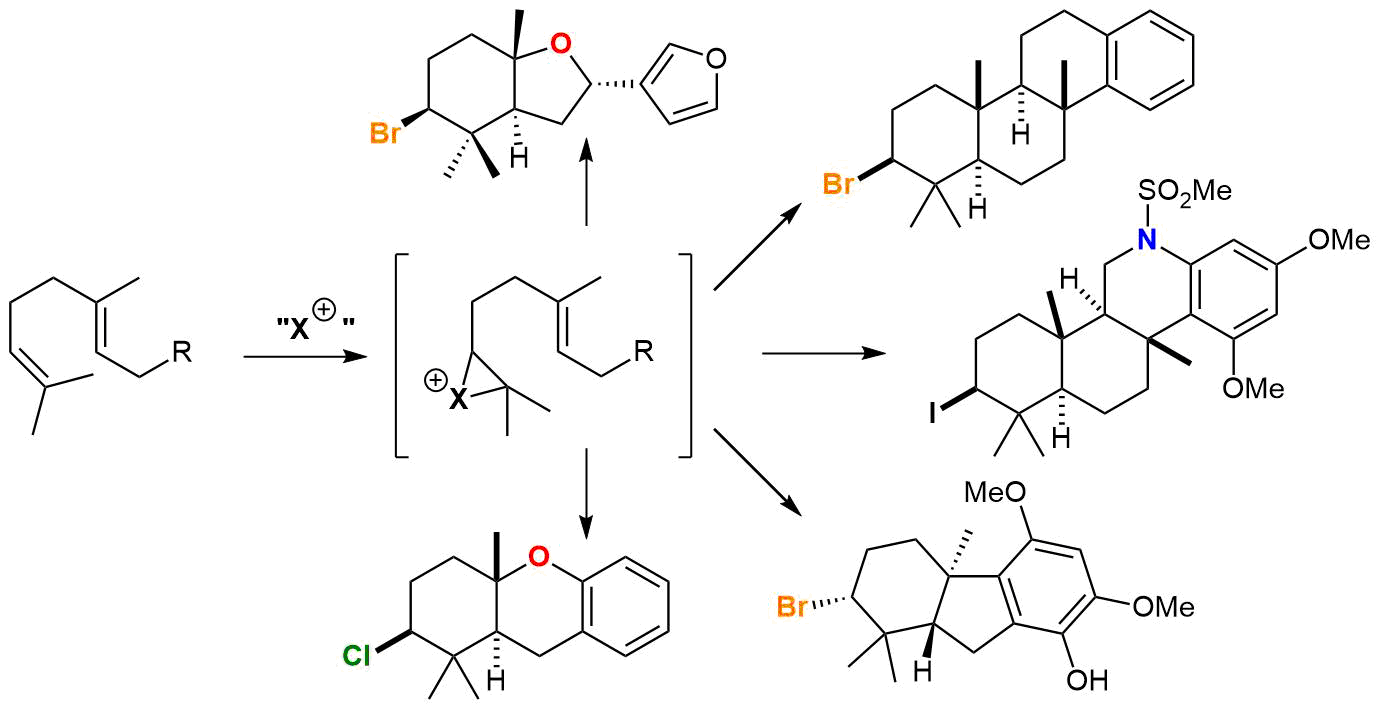
Diacetoxyiodo)benzene‐mediated Selective Synthesis of α‐Azido Ketones or Acyl Azides from β‐Keto Acids - Zheng - 2018 - Asian Journal of Organic Chemistry - Wiley Online Library

Silver(I)-catalyzed reaction of terminal alkynes with (diacetoxyiodo)benzene: a convenient, efficient and clean preparation of α-acetoxy ketones - ScienceDirect

Oxidation of 3,4-dihydropyrimidin-2(1H)-thione using (diacetoxyiodo)benzene: unprecedented formation of substituted 2-(1,4-dihydropyrimidin-2-ylthio)pyrimidine - ScienceDirect
![Novel preparation of 2,1-benzothiazine derivatives from sulfonamides with [hydroxy(tosyloxy)iodo]arenes - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/B301330H Novel preparation of 2,1-benzothiazine derivatives from sulfonamides with [hydroxy(tosyloxy)iodo]arenes - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/B301330H](https://pubs.rsc.org/image/article/2003/OB/b301330h/b301330h-s2.gif)
Novel preparation of 2,1-benzothiazine derivatives from sulfonamides with [hydroxy(tosyloxy)iodo]arenes - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/B301330H

Diacetoxyiodo)benzene‐mediated Selective Synthesis of α‐Azido Ketones or Acyl Azides from β‐Keto Acids - Zheng - 2018 - Asian Journal of Organic Chemistry - Wiley Online Library
![Molecules | Free Full-Text | Application of [Hydroxy(tosyloxy)iodo]benzene in the Wittig-Ring Expansion Sequence for the Synthesis of β-Benzocyclo-alkenones from α-Benzocycloalkenones | HTML Molecules | Free Full-Text | Application of [Hydroxy(tosyloxy)iodo]benzene in the Wittig-Ring Expansion Sequence for the Synthesis of β-Benzocyclo-alkenones from α-Benzocycloalkenones | HTML](https://www.mdpi.com/molecules/molecules-10-00217/article_deploy/html/images/molecules-10-00217-g001-550.jpg)
Molecules | Free Full-Text | Application of [Hydroxy(tosyloxy)iodo]benzene in the Wittig-Ring Expansion Sequence for the Synthesis of β-Benzocyclo-alkenones from α-Benzocycloalkenones | HTML
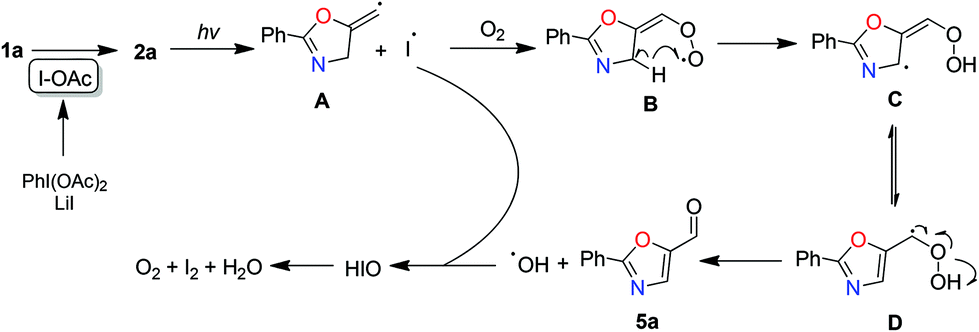
Preparation of oxazolines and oxazoles via a PhI(OAc)2-promoted cyclization of N-propargylamides - Organic & Biomolecular Chemistry (RSC Publishing)

Figure 1 from (Diacetoxyiodo)benzene-Mediated Reaction of Ethynylcarbinols: Entry to α,α'-Diacetoxy Ketones and Glycerol Derivatives. | Semantic Scholar
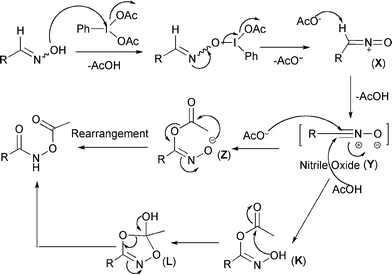
Hypervalent iodine(iii)-mediated oxidation of aldoximes to N-acetoxy or N-hydroxy amides - Organic & Biomolecular Chemistry (RSC Publishing)
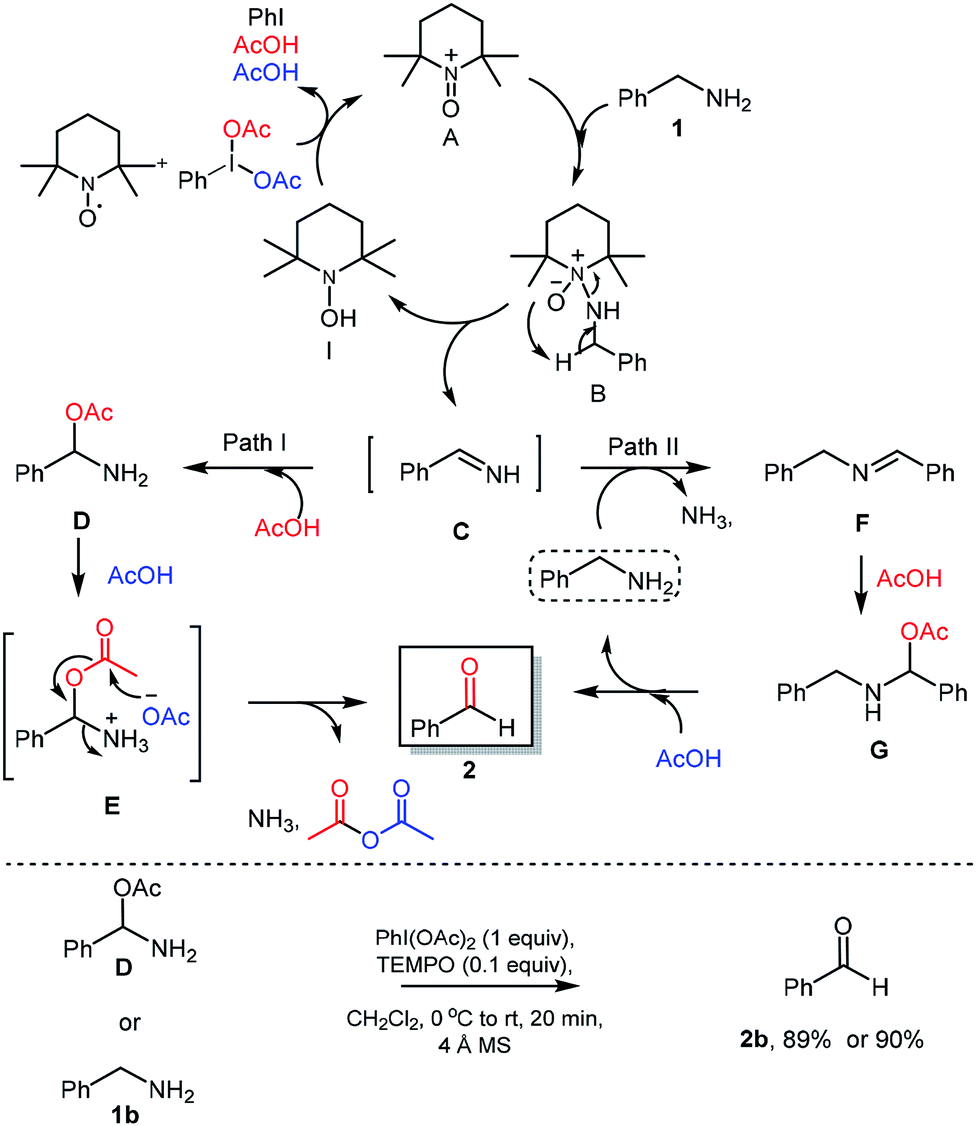
Metal-free hypervalent iodine/TEMPO mediated oxidation of amines and mechanistic insight into the reaction pathways - RSC Advances (RSC Publishing) DOI:10.1039/C8RA07451H

Mild propargylic oxidation using a diacetoxyiodobenzene/tert-butyl hydroperoxide protocol - ScienceDirect

Table 2 from (Diacetoxyiodo)benzene-Mediated Reaction of Ethynylcarbinols: Entry to α,α'-Diacetoxy Ketones and Glycerol Derivatives. | Semantic Scholar

Reaction of (diacetoxyiodo)benzene with excess of trifluoromethanesulfonic acid. A convenient route to para-phenylene type hypervalent iodine oligomers - ScienceDirect