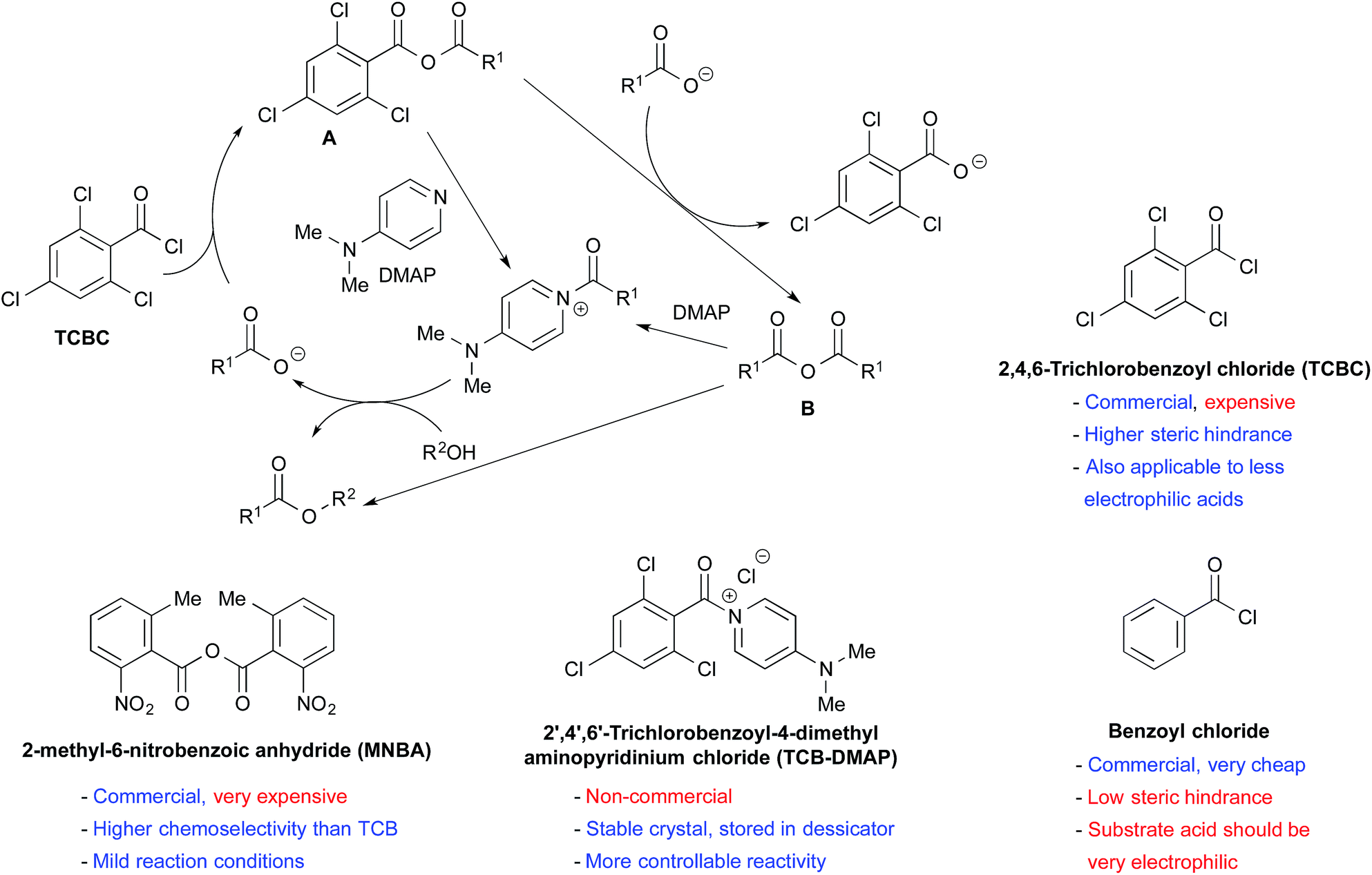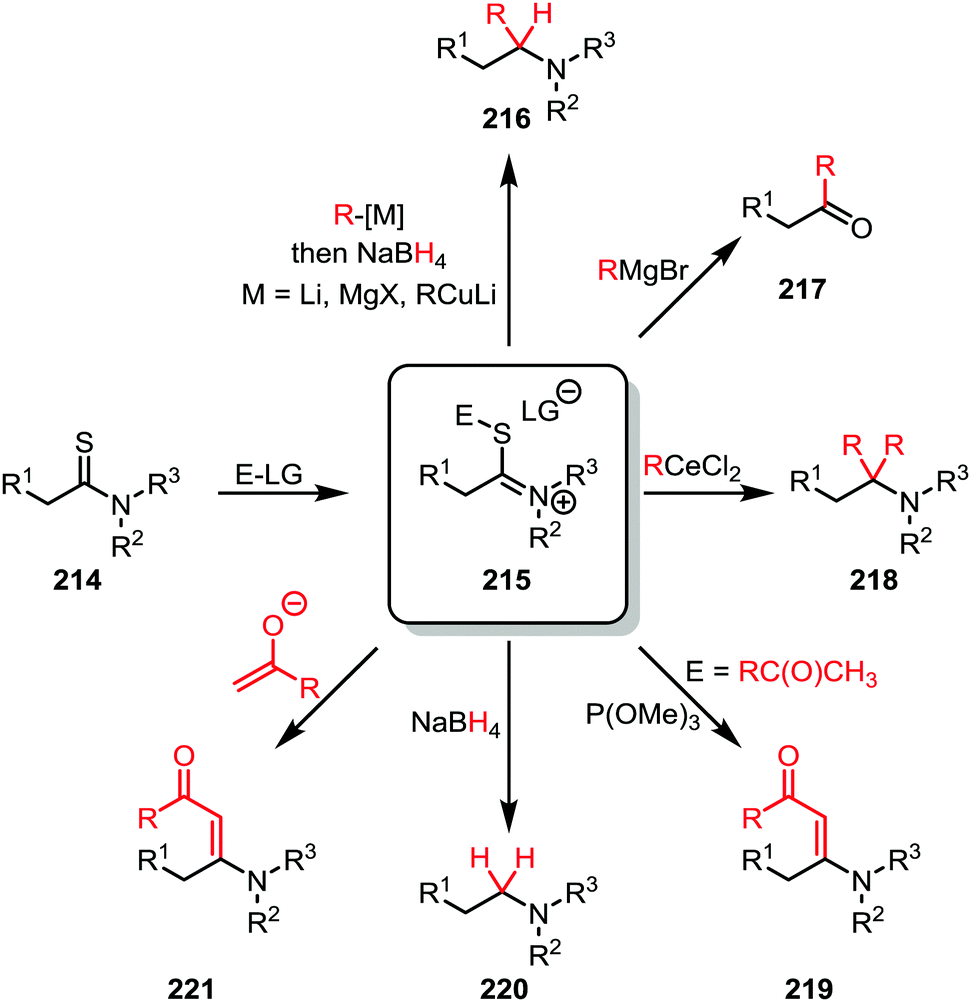
Amide activation: an emerging tool for chemoselective synthesis - Chemical Society Reviews (RSC Publishing)
![Flow synthesis of cyclobutanones via [2 + 2] cycloaddition of keteneiminium salts and ethylene gas - Reaction Chemistry & Engineering (RSC Publishing) DOI:10.1039/C7RE00020K Flow synthesis of cyclobutanones via [2 + 2] cycloaddition of keteneiminium salts and ethylene gas - Reaction Chemistry & Engineering (RSC Publishing) DOI:10.1039/C7RE00020K](https://pubs.rsc.org/image/article/2017/RE/c7re00020k/c7re00020k-s1_hi-res.gif)
Flow synthesis of cyclobutanones via [2 + 2] cycloaddition of keteneiminium salts and ethylene gas - Reaction Chemistry & Engineering (RSC Publishing) DOI:10.1039/C7RE00020K
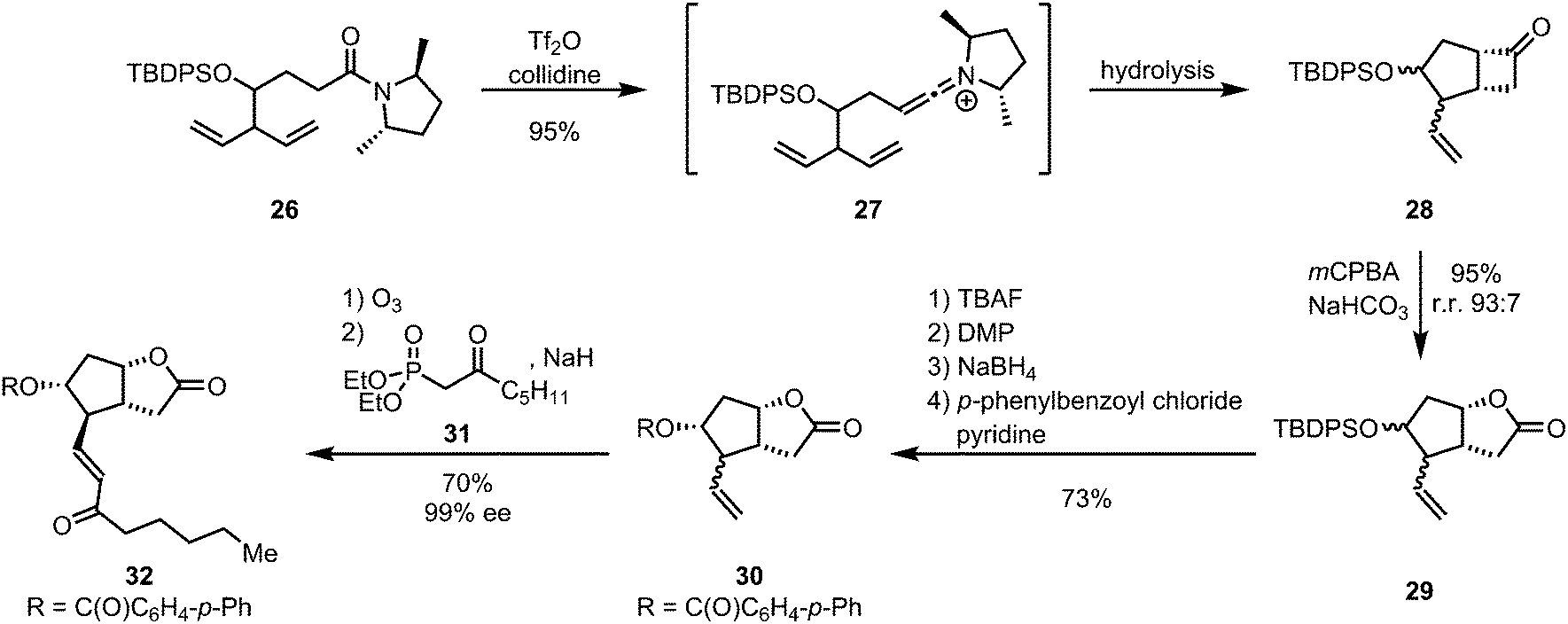
Amide activation: an emerging tool for chemoselective synthesis - Chemical Society Reviews (RSC Publishing)

Recent Advances in Minisci‐Type Reactions - Proctor - 2019 - Angewandte Chemie International Edition - Wiley Online Library
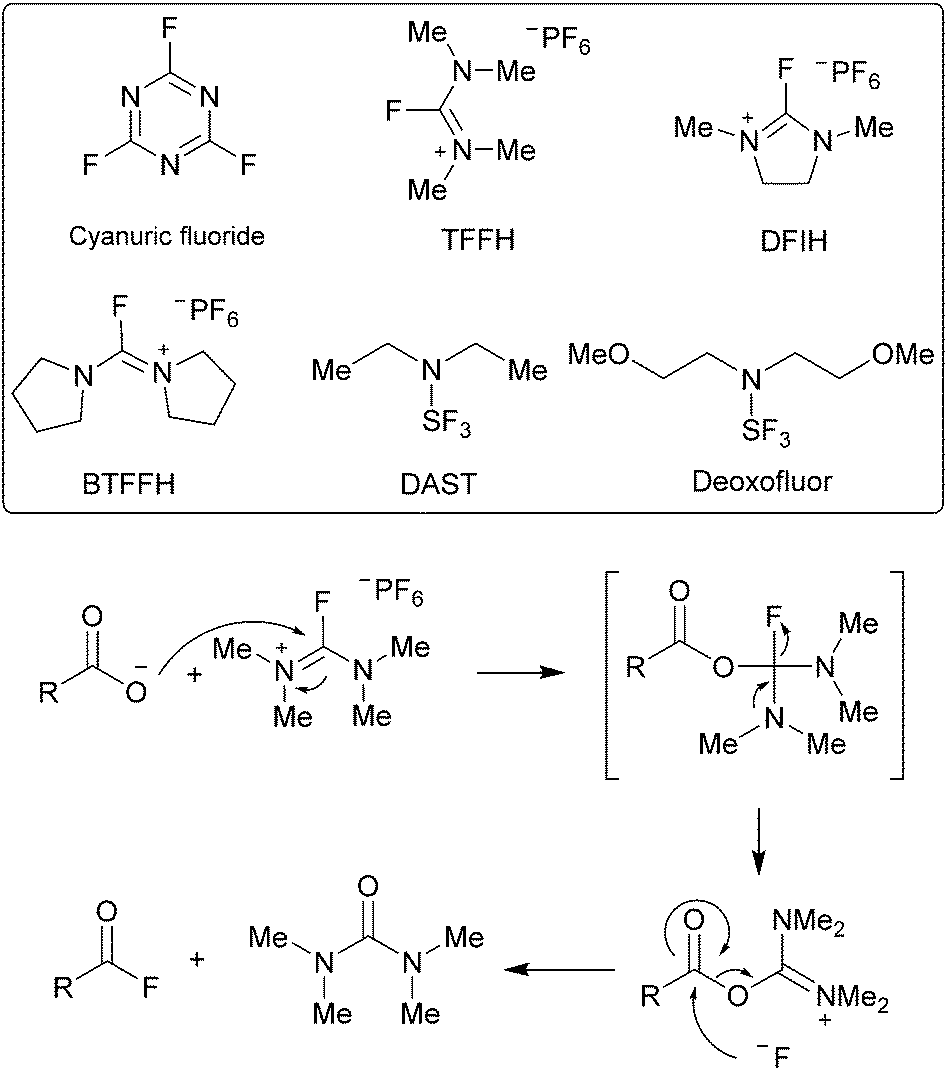
Ester coupling reactions – an enduring challenge in the chemical synthesis of bioactive natural products - Natural Product Reports (RSC Publishing)

PDF) Studies of Palladium-Catalyzed Coupling Reactions for Preparation of Hindered 3-Arylpyrroles Relevant to (-)-Rhazinilam and Its Analogues

Solid-phase methodology for synthesis of O-alkylated aromatic oligoamide inhibitors of α-helix-mediated protein-protein interactions. - Abstract - Europe PMC