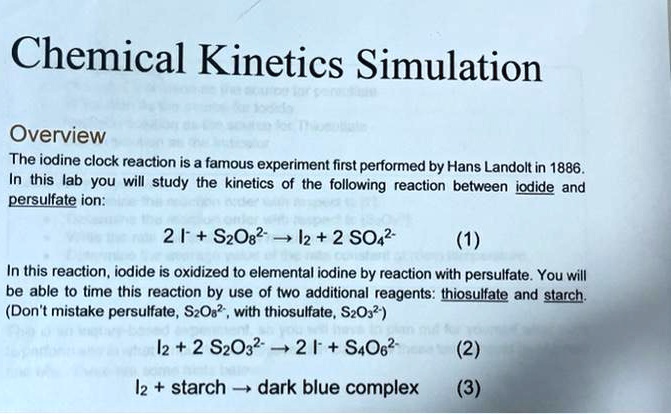
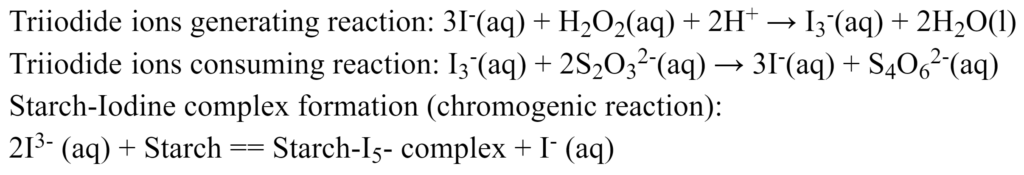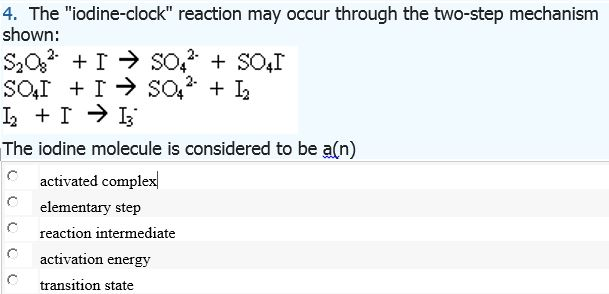The general reaction scheme for an iodine clock reaction: (red) species... | Download Scientific Diagram

Iodine Clock Reaction We will begin by describing a proposed reaction mechanism for the iodine clock reaction. There are several variations to this reaction, - ppt download

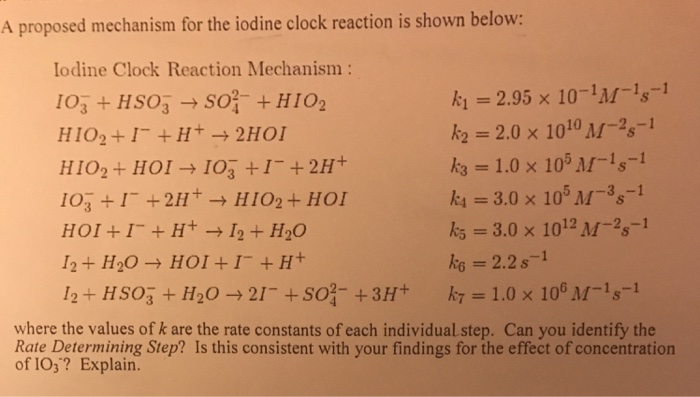
SOLVED:proposed mechanism for the iodine clock reaction is shown below: Iodine Clock Reaction Mechanism IO3 + HSO3 S0} + HIO2 k =2.95 x 10-'M-Is-1 HIO2 + I- + H+ + 2HOI k2 =

PPT - Rainer Glaser, Chemistry MLS Proseminar , November 16, 2009 (with updates) PowerPoint Presentation - ID:2281042

Student Name: Instructor Name: EXP 4: POST-LAB 1. A proposed mechanism for the iodine clock reaction... - HomeworkLib
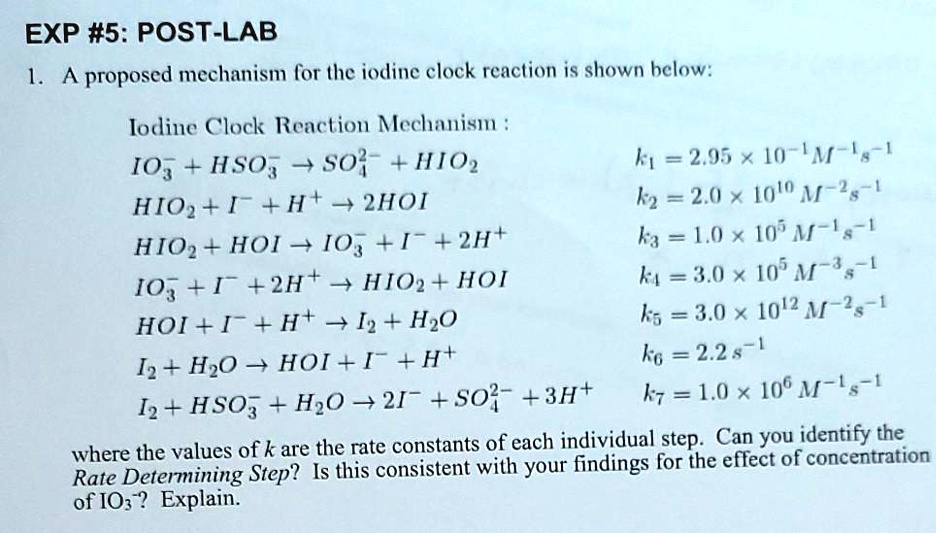
SOLVED:EXP #5: POST-LAB proposed mechanism lor (he iodine clock reaction is shown helow: Iodline Clock Reaction Mechanism 4 S0; +HO2 =2.95 x 10-'M-!,-[ IO, HSO; HIO2 + 1 + H+ 3 2HOI
![A clock reaction is run at 20 �C with sev[{Image src='img20401705385059449876496.jpg' alt='' caption=''}]eral different mixtures of iodide, sodium bromate and acid, to form iodine. Thiosulfate is used | Study.com A clock reaction is run at 20 �C with sev[{Image src='img20401705385059449876496.jpg' alt='' caption=''}]eral different mixtures of iodide, sodium bromate and acid, to form iodine. Thiosulfate is used | Study.com](https://study.com/cimages/multimages/16/img20401705385059449876496.jpg)
A clock reaction is run at 20 �C with sev[{Image src='img20401705385059449876496.jpg' alt='' caption=''}]eral different mixtures of iodide, sodium bromate and acid, to form iodine. Thiosulfate is used | Study.com

How does the activation enthalpy and the rate of the iodine-clock reaction vary with the concentration of reactants, catalysts, and the presence of different catalysts? - GCSE Science - Marked by Teachers.com

A Closer Examination of the Mechanism of the Hydrogen Peroxide Iodine-Clock Reaction with Respect to the Role of Hypoiodite Species,Journal of Chemical Education - X-MOL
![Rate Law Equation For the reaction: aX + bY products r = k [X] m [Y] n Where r = reaction rate k = rate constant [X] & [Y] = concentration Rate Law Equation For the reaction: aX + bY products r = k [X] m [Y] n Where r = reaction rate k = rate constant [X] & [Y] = concentration](https://images.slideplayer.com/25/8124398/slides/slide_5.jpg)
Rate Law Equation For the reaction: aX + bY products r = k [X] m [Y] n Where r = reaction rate k = rate constant [X] & [Y] = concentration