
Biomimetic alkane oxidation by iodosylbenzene and iodobenzene diacetate catalyzed by a new manganese porphyrin: Water effect - ScienceDirect

A biomimetic oxidation catalyzed by manganese(III) porphyrins and iodobenzene diacetate: Synthetic and mechanistic investigations - ScienceDirect
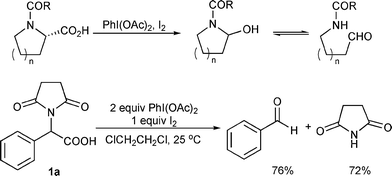
A one-pot oxidative decarboxylation–Friedel-Crafts reaction of acyclic α-amino acid derivatives activated by the combination of iodobenzene diacetate ... - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/B815227F

organic chemistry - Mechanism of oxidative dearomatisation with hypervalent iodine - Chemistry Stack Exchange

PDF) ChemInform Abstract: Regiospecific Oxidation of Polycyclic Aromatic Phenols to Quinones by Hypervalent Iodine Reagents

organic chemistry - Mechanism of oxidative dearomatisation with hypervalent iodine - Chemistry Stack Exchange

Synthesis of 3,5-diarylisoxazoles under solvent-free conditions using iodobenzene diacetate - ScienceDirect

A one-pot oxidative decarboxylation–Friedel-Crafts reaction of acyclic α-amino acid derivatives activated by the combination of iodobenzene diacetate ... - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/B815227F

Fused dihydrofurans from the one-pot, three-component reaction of 1,3-cyclohexanedione, iodobenzene diacetate and alkenes - ScienceDirect














