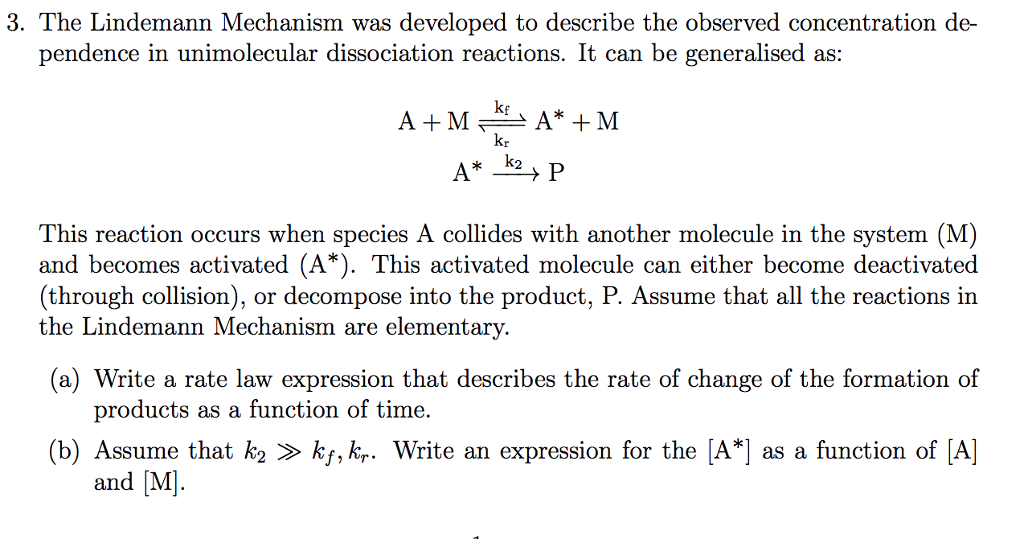
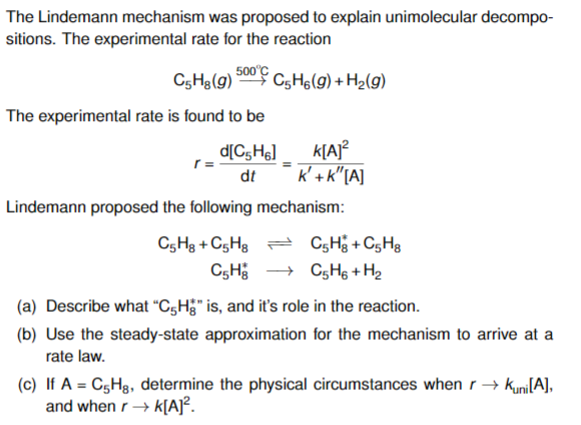
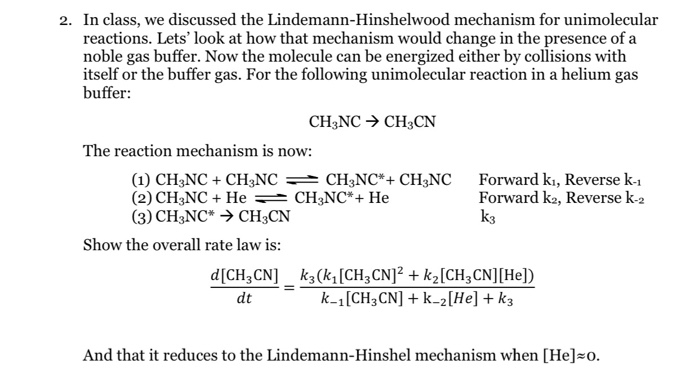
Chemistry 232 Complex Reaction Mechanisms. Lindemann-Hinshelwood Mechanism An early attempt to explain the kinetics of complex reactions. Mechanism Rate. - ppt download
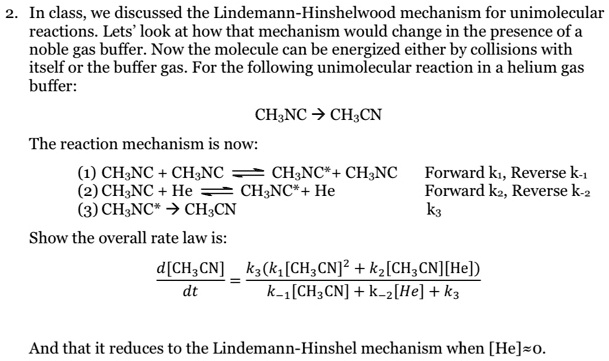
SOLVED:In class_ we discussed the Lindemann-Hinshelwood mechanism for unimolecular reactions. Lets' look at how that mechanism would change in the presence of a noble gas buffer: Now the molecule can be energized
![SOLVED:Find the rate law expression for the following proposed Lindemann mechanism ofa reactant A in the presence of an inert molecule M when the [M] = 0. A+M A* +M A +A A* + SOLVED:Find the rate law expression for the following proposed Lindemann mechanism ofa reactant A in the presence of an inert molecule M when the [M] = 0. A+M A* +M A +A A* +](https://cdn.numerade.com/ask_images/0c7779bfd1a24997b917a080935e5773.jpg)
SOLVED:Find the rate law expression for the following proposed Lindemann mechanism ofa reactant A in the presence of an inert molecule M when the [M] = 0. A+M A* +M A +A A* +
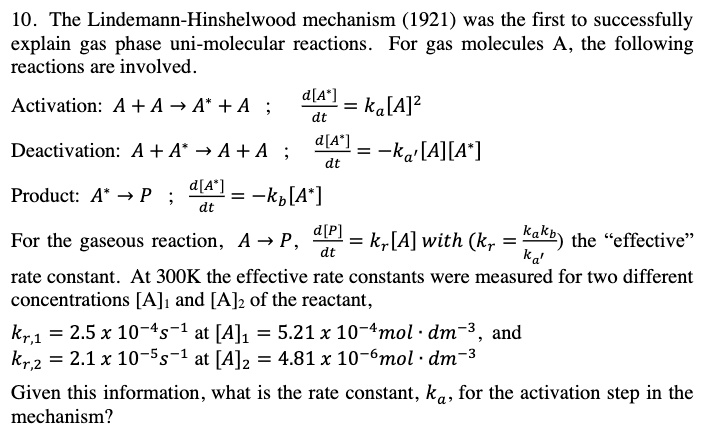
SOLVED:10 _ The Lindemann-Hinshelwood mechanism (1921) was the first to successfully explain gas phase uni-molecular reactions. For gas molecules A, the following reactions are involved. Activation: A + A +A* +A 44! =
Solved] 16b The effective rate constant for a gaseous reaction that has a Lindemann-Hinshelwood mechanism is 1.7 x 10 3s at 1.09 kPa and 2.2 x 10 #s... | Course Hero
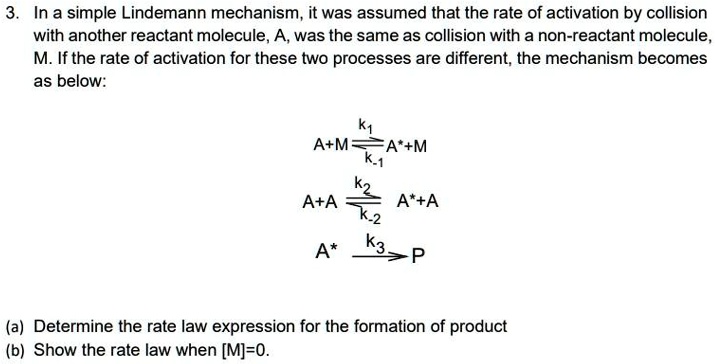
SOLVED:In a simple Lindemann mechanism, it was assumed that the rate of activation by collision with another reactant molecule_ A, was the same as collision with a non-reactant molecule_ M: If the
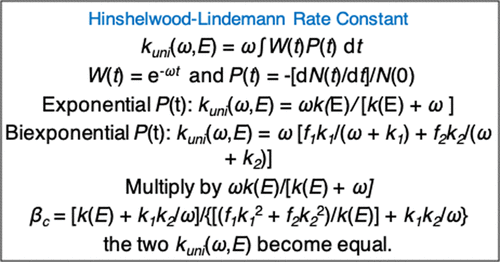






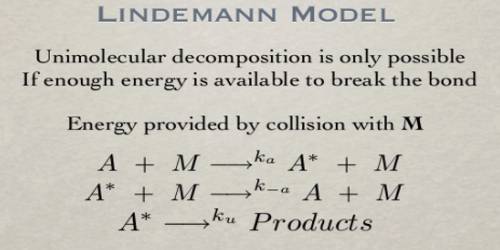



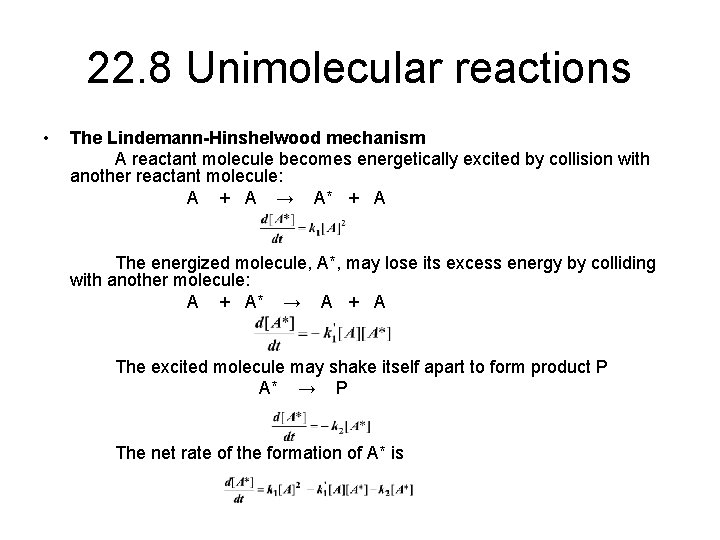


![PDF] Properties of the Lindemann Mechanism in Phase Space | Semantic Scholar PDF] Properties of the Lindemann Mechanism in Phase Space | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/15490323f7a8b3887c7922c8303f17426afcd22e/11-Figure4.1-1.png)