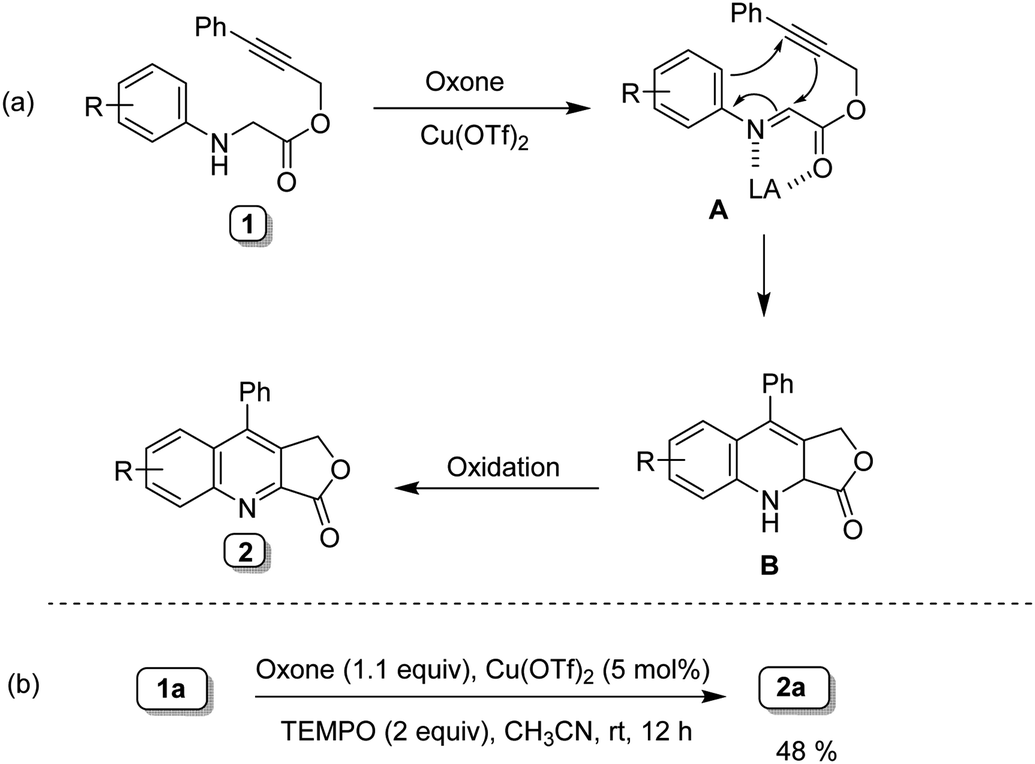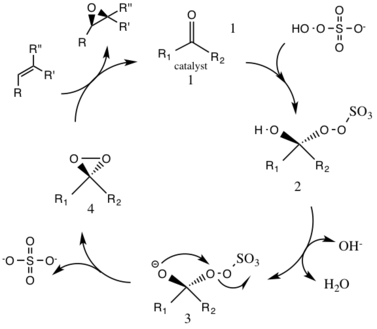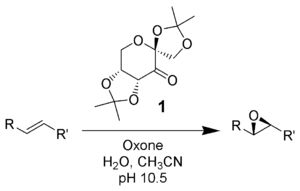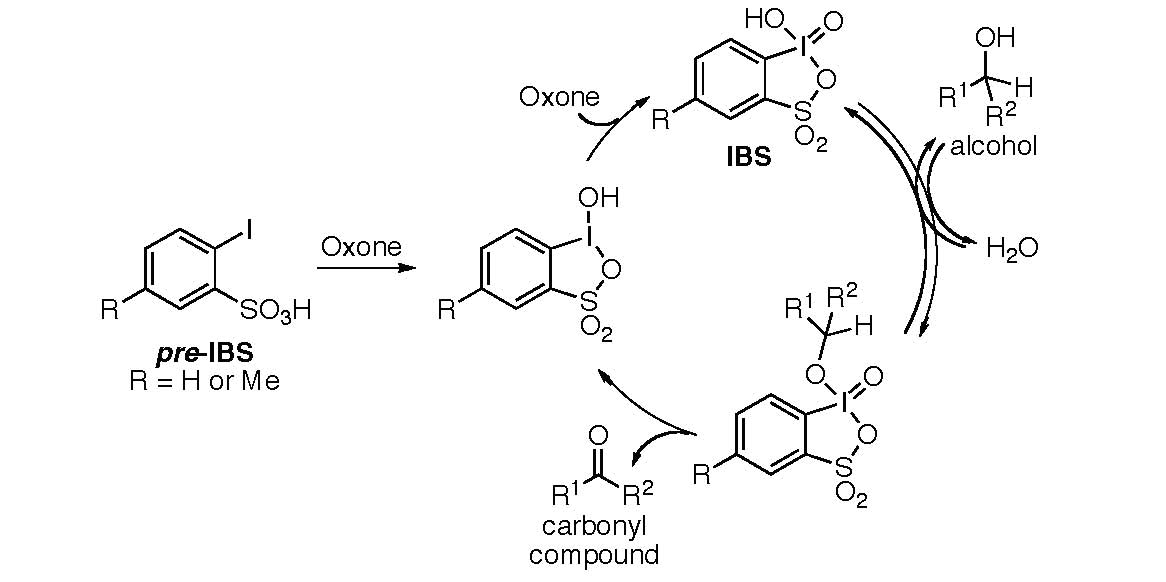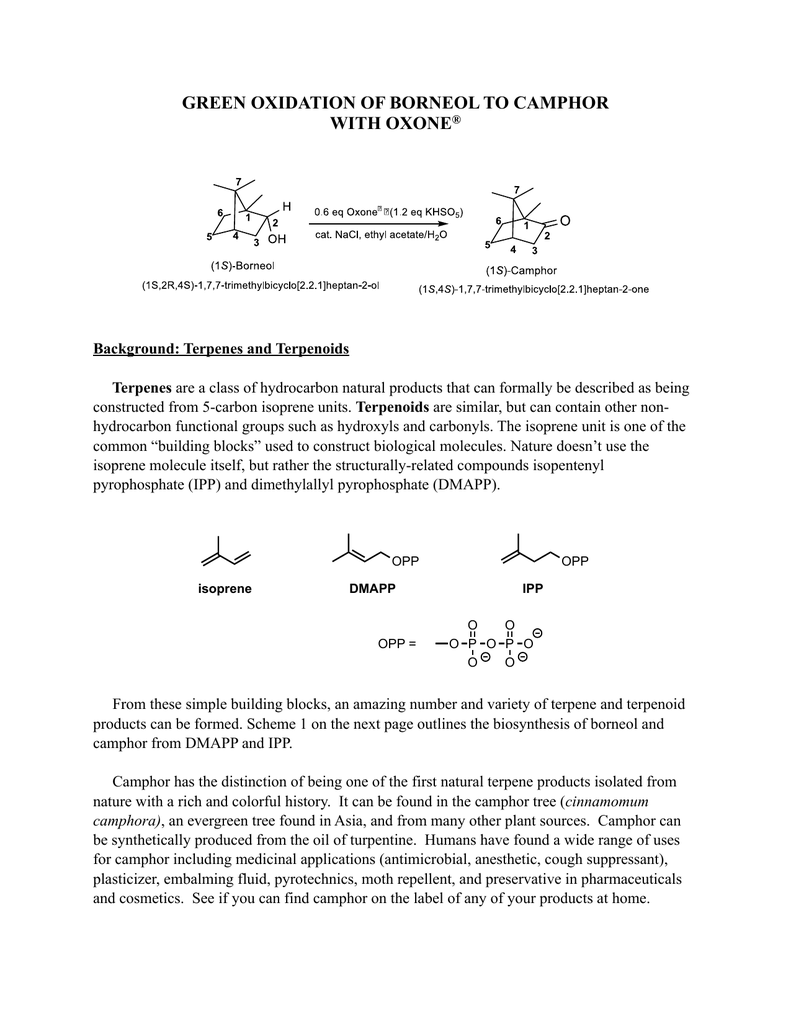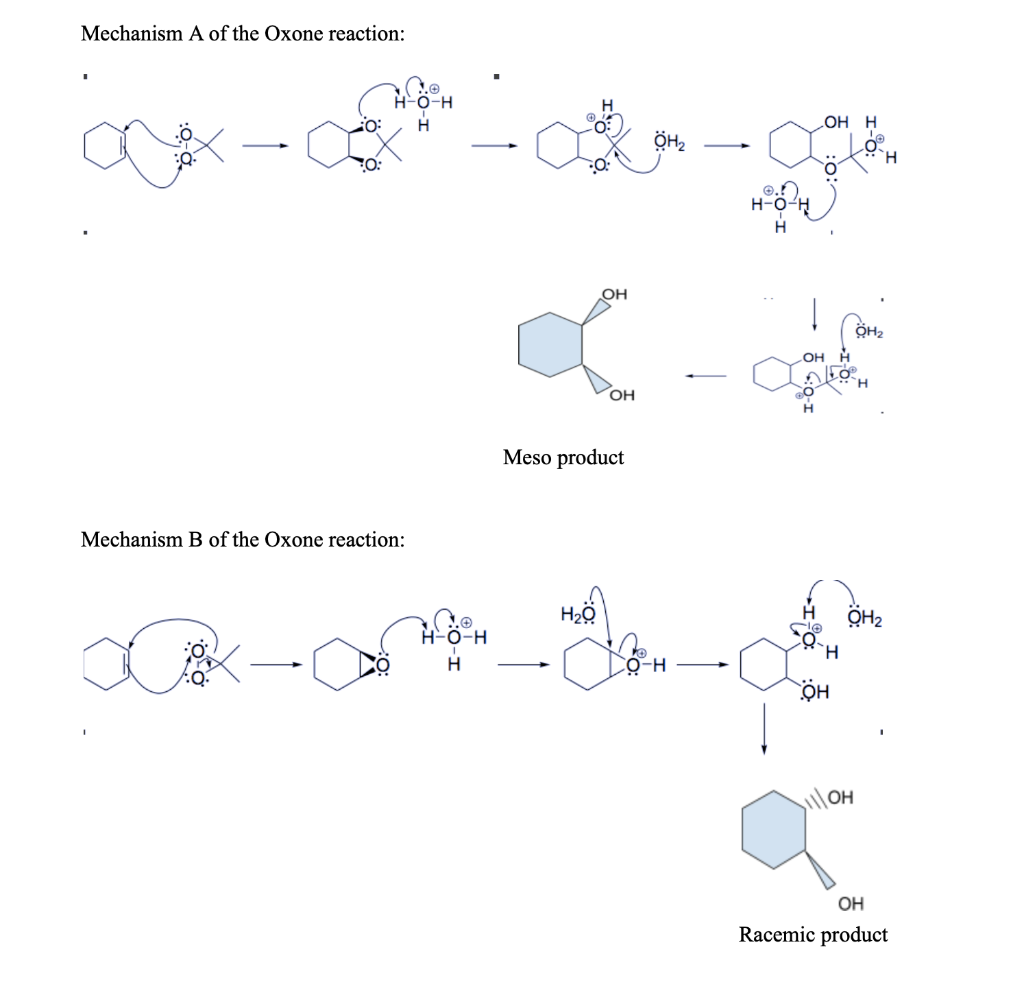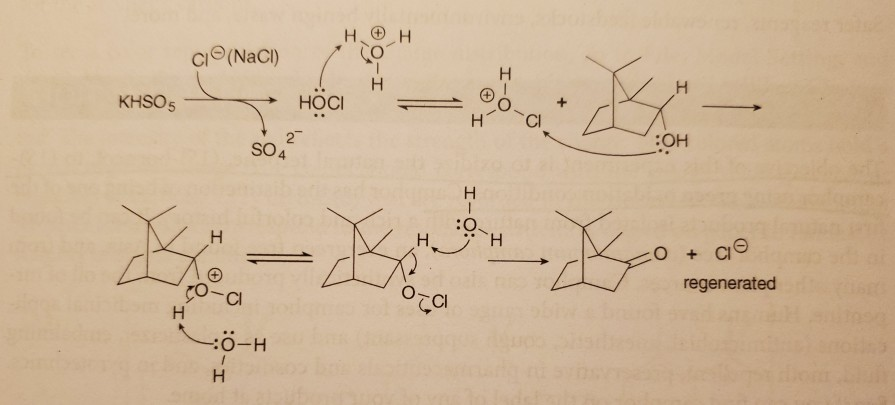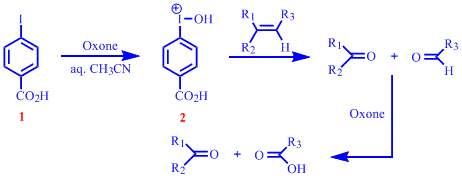
Report: Oxidation of Alkenes in Aqueous Solvent Mixtures Using Environmentally Benign Reagents (56th Annual Report on Research Under Sponsorship of The American Chemical Society Petroleum Research Fund)

Catalyst-free oxidation of sulfides to sulfoxides and diethylamine-catalyzed oxidation of sulfides to sulfones using Oxone as an oxidant | Request PDF

Plausible mechanism for diethylamine-catalyzed oxidation of sulfides to... | Download Scientific Diagram
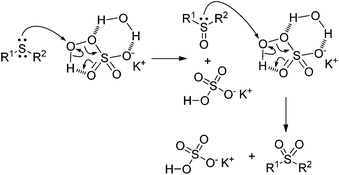
Catalyst -free approach for solvent -dependent selective oxidation of organic sulfides with oxone - Green Chemistry (RSC Publishing) DOI:10.1039/C2GC00027J

Amine‐Catalyzed Epoxidation of Alkenes: A New Mechanism for the Activation of Oxone - Armstrong - 2004 - Angewandte Chemie International Edition - Wiley Online Library

Facile synthesis of symmetric thiosulfonates by oxidation of disulfide with oxone/MX (MX = KBr, KCl, NaBr and NaCl) - ScienceDirect
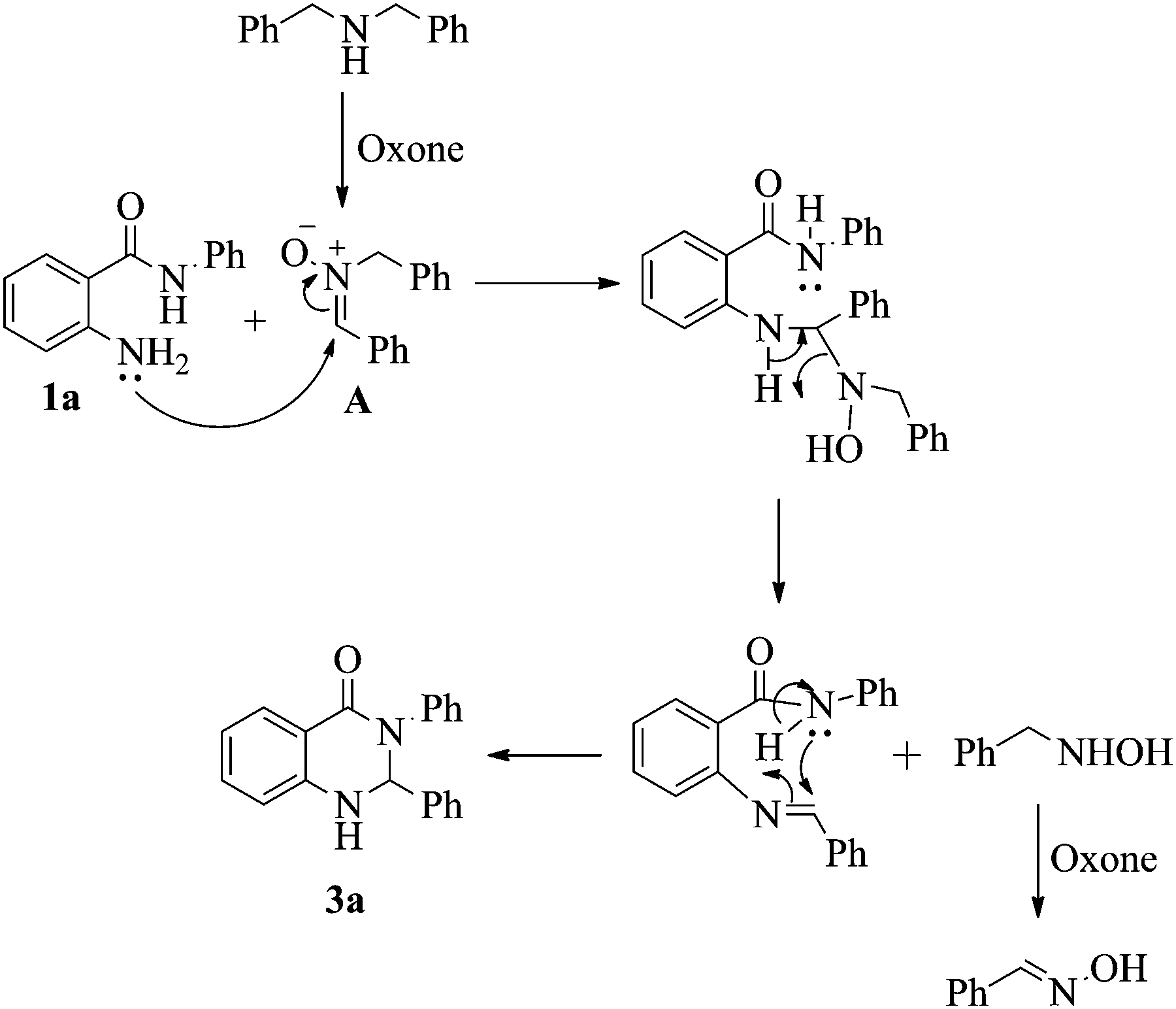
Oxone-mediated annulation of 2-aminobenzamides and 1,2-diaminobenzenes with sec -amines via imine- N -oxides: new syntheses of 2,3-dihydroquinazolin-4 ... - New Journal of Chemistry (RSC Publishing) DOI:10.1039/C7NJ04939K

organic chemistry - Mechanism for the conversion of 4-bromobenzyl bromide to 4-bromobenzoic acid using Oxone (potassium peroxymonosulfate)? - Chemistry Stack Exchange
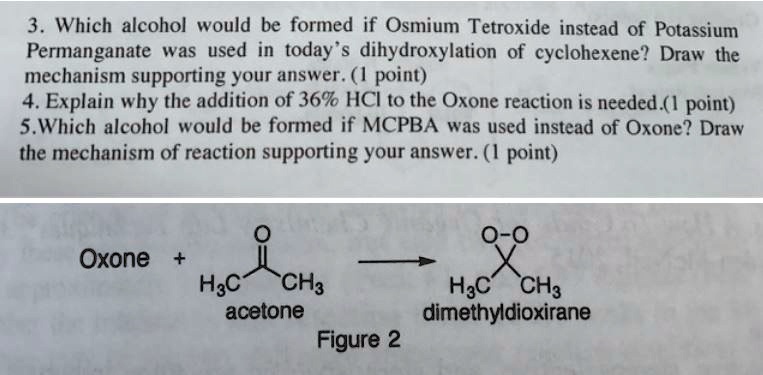
SOLVED:3 . Which alcohol would be formed if Osmium Tetroxide instead of Potassium Permanganate was used in today's dihydroxylation of cyclohexene? Draw the mechanism supporting your answer. ( 1 point) 4.Explain why
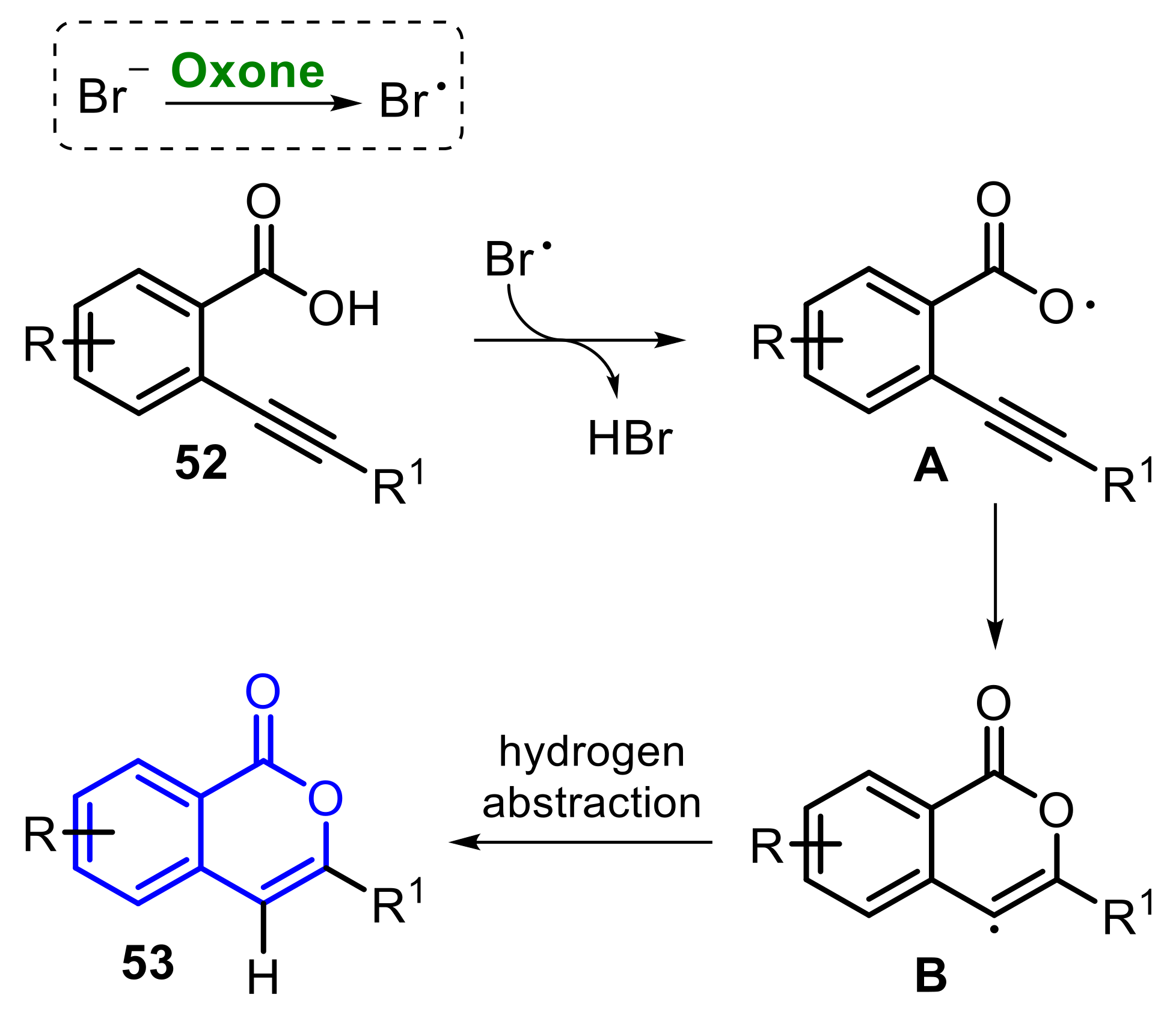
Molecules | Free Full-Text | Recent Advances in the Oxone-Mediated Synthesis of Heterocyclic Compounds