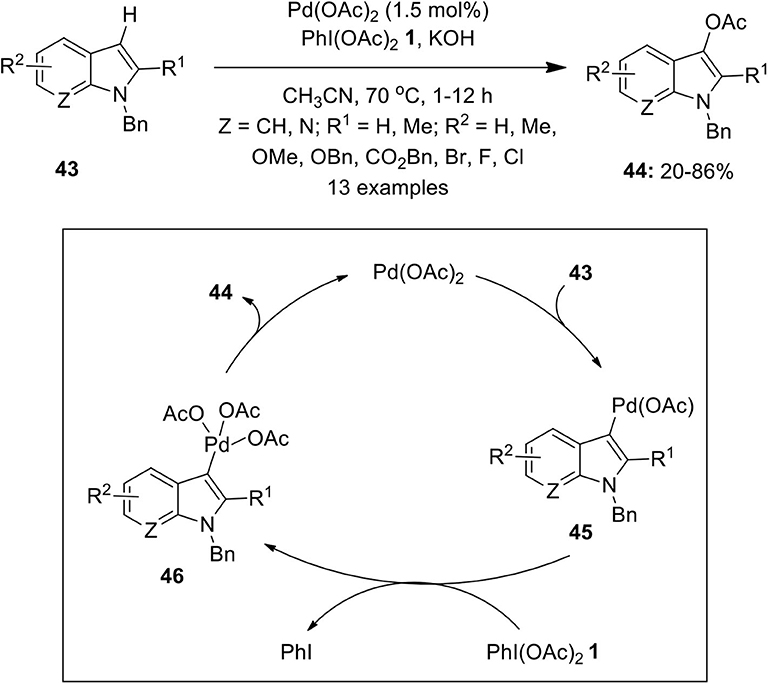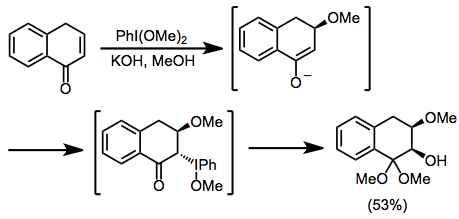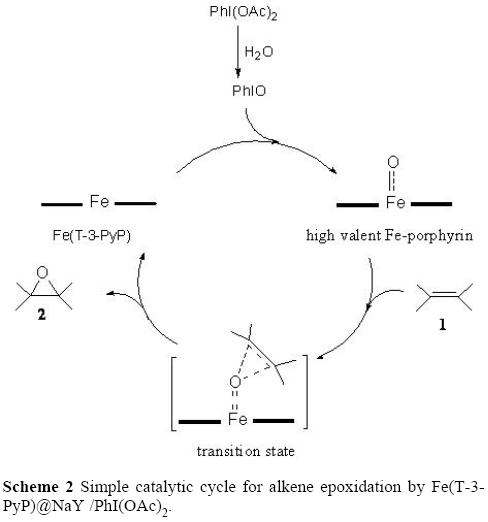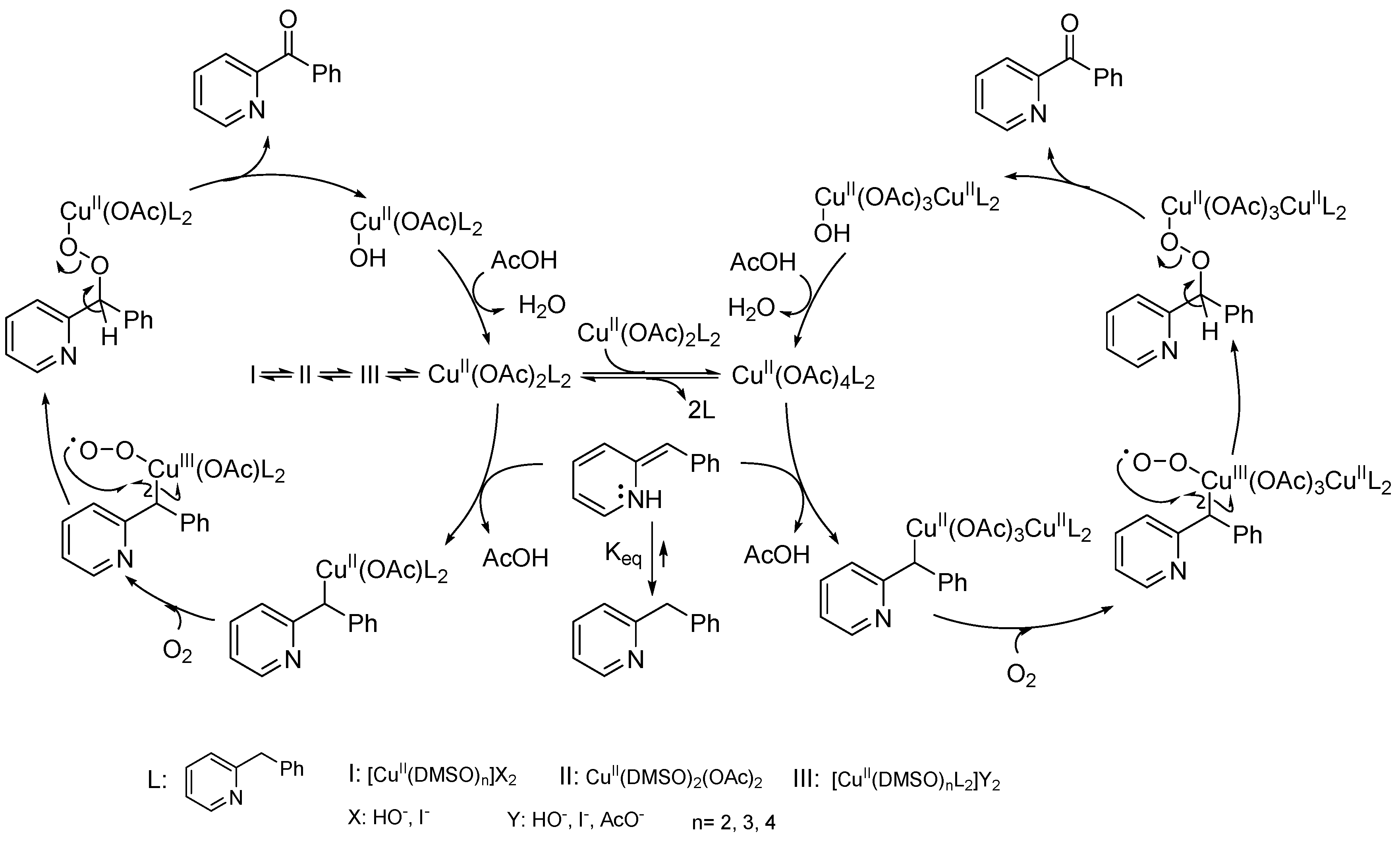
Catalysts | Free Full-Text | Recent Advances in Homogeneous Metal-Catalyzed Aerobic C–H Oxidation of Benzylic Compounds | HTML

The reaction of terminal alkynes with PhI(OAc)2: a convenient procedure for the preparation of α-acyloxy ketones - ScienceDirect

Molecules | Free Full-Text | Hypervalent Iodine Reagents in High Valent Transition Metal Chemistry | HTML

PDF) Nitrate as a Redox Co-Catalyst for the Aerobic Pd-Catalyzed Oxidation of Unactivated sp(3)-C-H Bonds
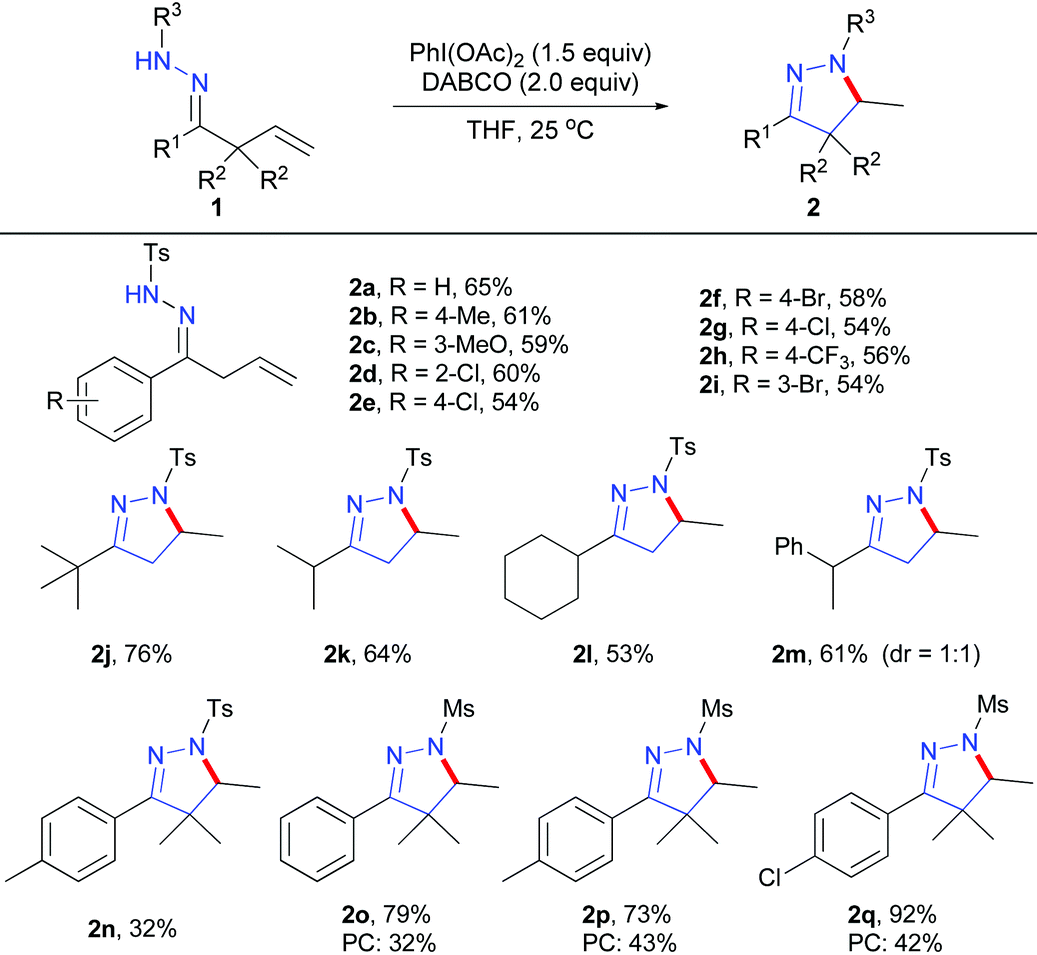
PhI(OAc)2-mediated functionalisation of unactivated alkenes for the synthesis of pyrazoline and isoxazoline derivatives - Organic & Biomolecular Chemistry (RSC Publishing)

Benoît Moreau Organohypervalent Iodine as Mild and Selective Reagents for Multiple Oxidation Processes Literature Meeting June 6 th, ppt download

Practical application of PhI(OAc)2/I2 combination to synthesize benzimidazoles from 2-aminobenzylamine through ring distortion strategy - ScienceDirect

Copper‐Catalyzed Decarboxylative Methylation of Aromatic Carboxylic Acids with PhI(OAc)2 - Jiang - 2014 - European Journal of Organic Chemistry - Wiley Online Library

Catalytic hypervalent iodine oxidation of alcohols to corresponding aldehydes or ketones using 2,2,6,6-tetramethylpiperidinyl-1-oxy and potassium peroxodisulfate | SpringerLink

Asymmetric oxidative dearomatizations promoted by hypervalent iodine(III) reagents: an opportunity for rational catalyst design? - ScienceDirect

Copper-Promoted Functionalization of Organic Molecules: from Biologically Relevant Cu/O2 Model Systems to Organometallic Transformations. - Abstract - Europe PMC

Frontiers | Hypervalent Iodine-Mediated Diastereoselective α-Acetoxylation of Cyclic Ketones | Chemistry

A highly efficient TEMPO mediated oxidation of sugar primary alcohols into uronic acids using 1-chloro-1,2-benziodoxol-3(1H)-one at room temperature - ScienceDirect