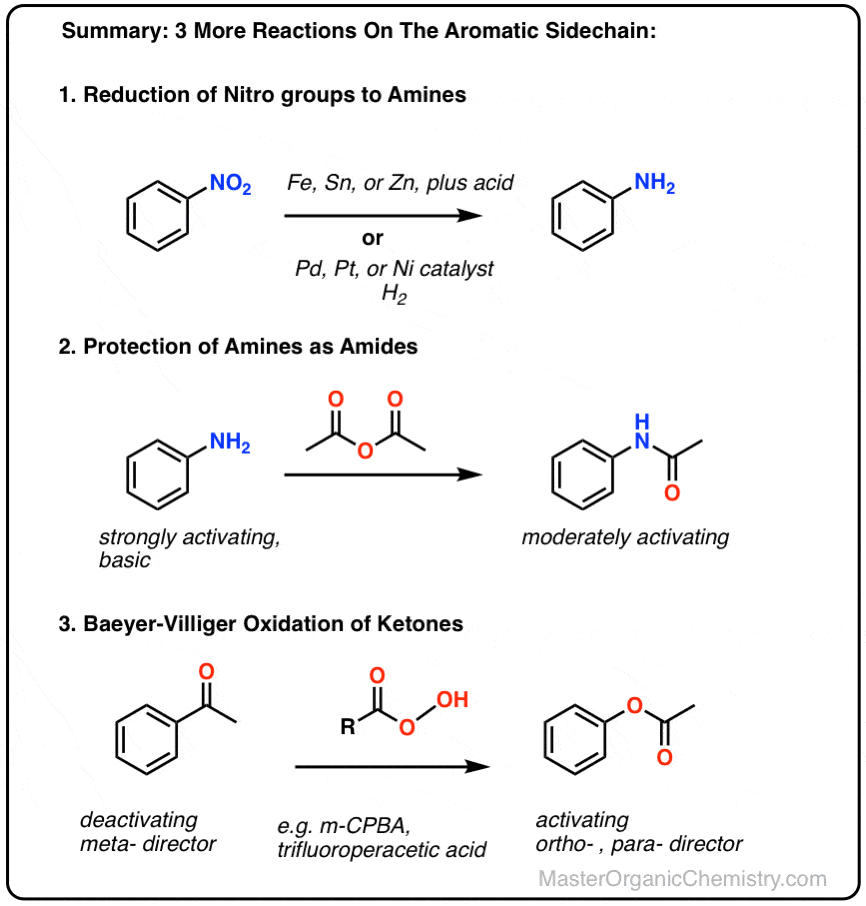
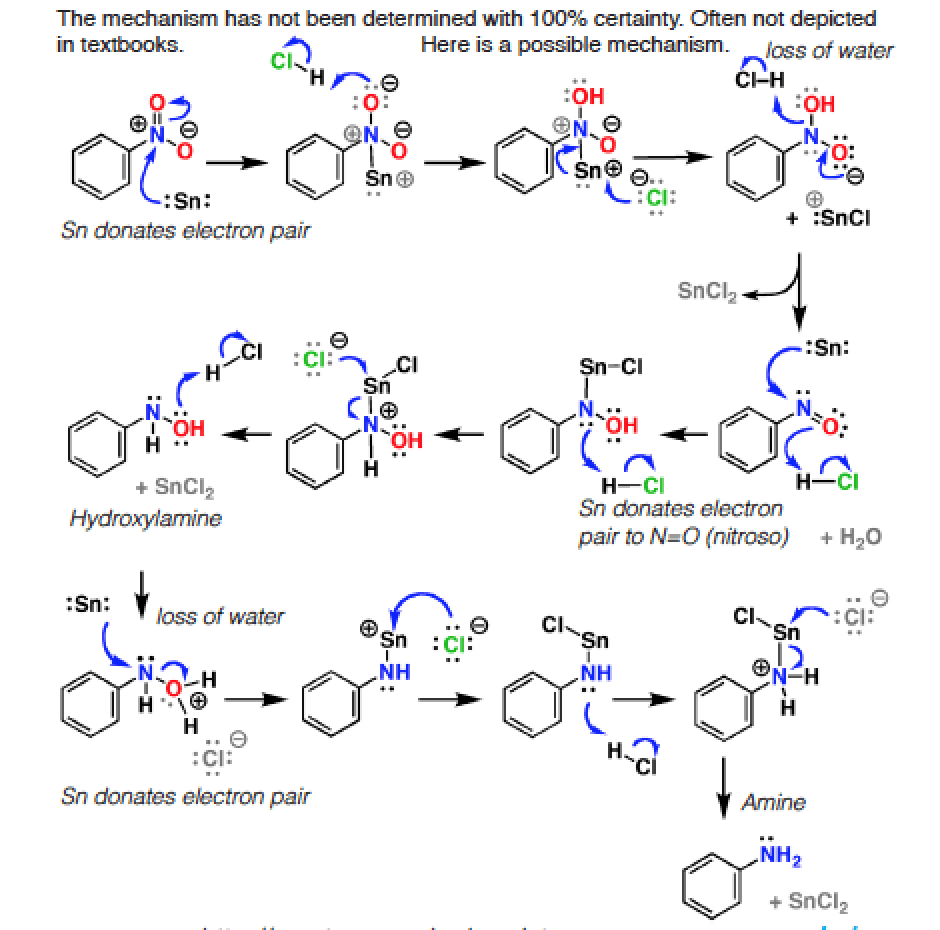
A DFT study of reduction of nitrobenzene to aniline with SnCl2 and hydrochloric acid - Yamabe - 2016 - Journal of Physical Organic Chemistry - Wiley Online Library

organic chemistry - Nitrobenzene reduction with (tin) Sn catalyst: Why is C-H bond cleavage preferred over O-H bond cleavage? - Chemistry Stack Exchange

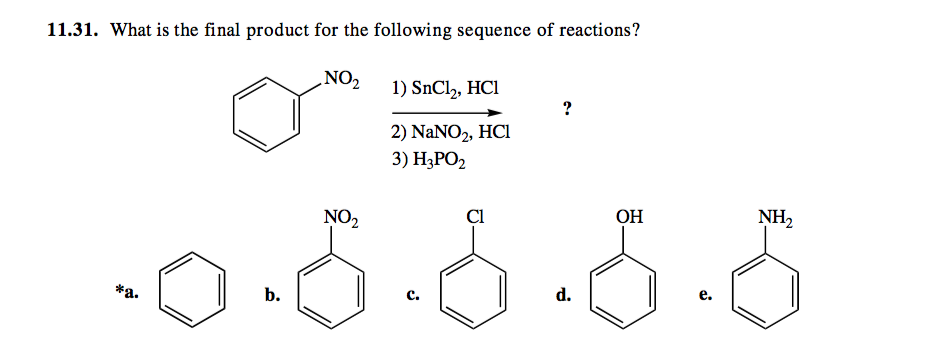
Scheme 1. Reagents and conditions: (a) NaNO2, HCl, SnCl2, -5 ⁰C to RT,... | Download Scientific Diagram

A DFT study of reduction of nitrobenzene to aniline with SnCl2 and hydrochloric acid - Yamabe - 2016 - Journal of Physical Organic Chemistry - Wiley Online Library

Scheme 1. Reagents and conditions: (a) NaNO2, HCl, SnCl2, -5 ⁰C to RT,... | Download Scientific Diagram

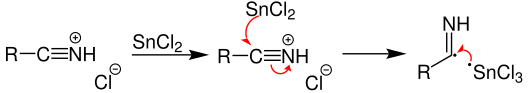
organic chemistry - Does this reduction mechanism of an diazonium via stannic chloride sense? - Chemistry Stack Exchange
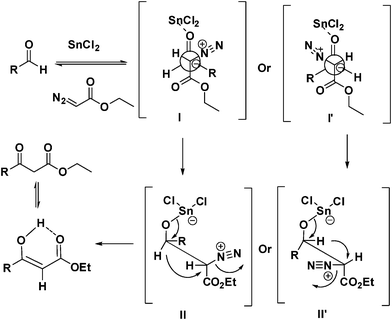
Tin(ii) chloride assisted synthesis of N -protected γ -amino β -keto esters through semipinacol rearrangement - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/C0OB00199F

A DFT study of reduction of nitrobenzene to aniline with SnCl2 and hydrochloric acid - Yamabe - 2016 - Journal of Physical Organic Chemistry - Wiley Online Library

Synthesis of 2,5-disubstitued benzimidazole using SnCl2-catalyzed reduction system at room temperature - ScienceDirect



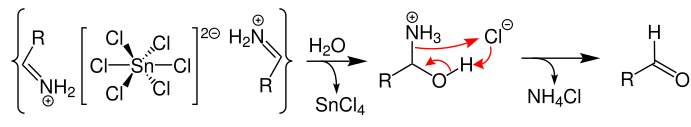




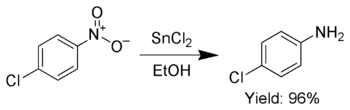

![The reaction RCN [ ]SnCl2/HCl (A) [ ]H2O RCHO + NH4Cl is known as: The reaction RCN [ ]SnCl2/HCl (A) [ ]H2O RCHO + NH4Cl is known as:](https://d1hj4to4g9ba46.cloudfront.net/questions/1645507_1780626_ans_cfe45c9ca8fe42bd9c9eb464a1a38714.png)