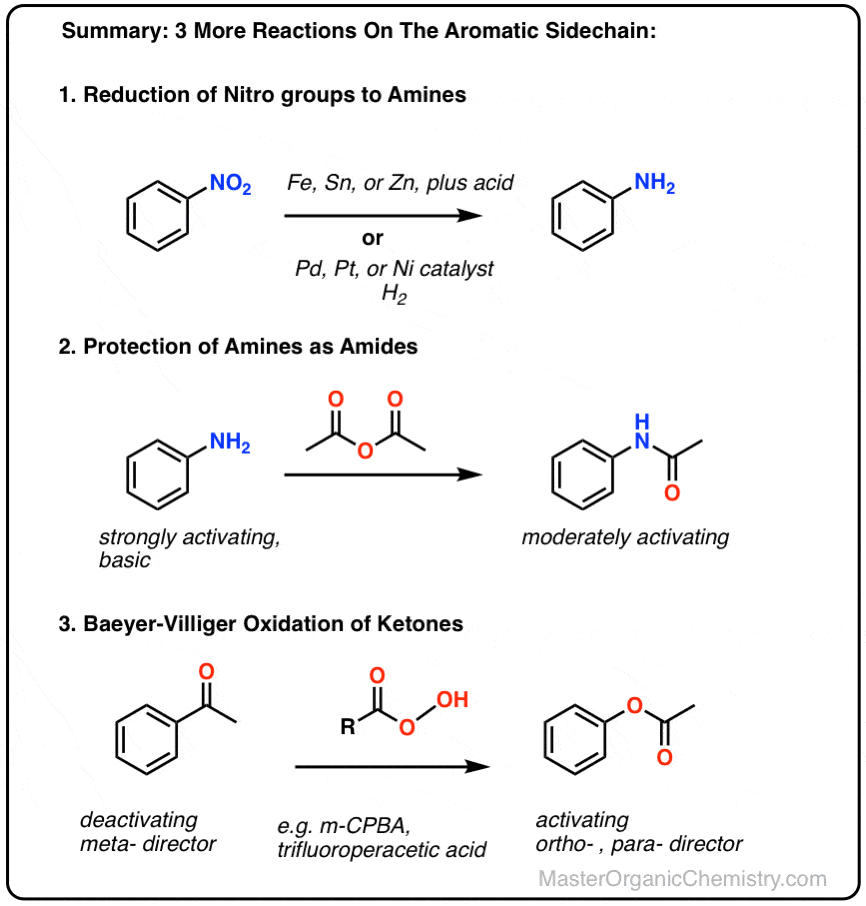
organic chemistry - Nitrobenzene reduction with (tin) Sn catalyst: Why is C-H bond cleavage preferred over O-H bond cleavage? - Chemistry Stack Exchange

organic chemistry - Pyridine synthesis by tin(II) chloride reduction of 5-nitronorbornene - Chemistry Stack Exchange

A DFT study of reduction of nitrobenzene to aniline with SnCl2 and hydrochloric acid - Yamabe - 2016 - Journal of Physical Organic Chemistry - Wiley Online Library
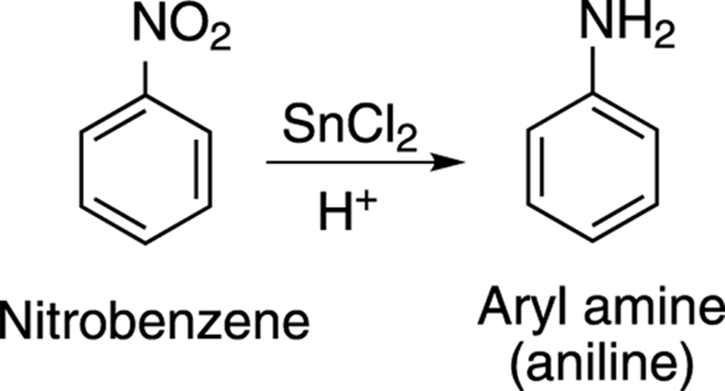
Bringing Out the Howitzers: Reactions of Aromatic Compounds - Functional Groups - Organic Chemistry I For Dummies, 2nd Edition (2014)

Facile synthesis of 2-substituted quinolines and 3-alkynyl-2-aryl-2 H -Indazole via SnCl 2 -mediated reductive cyclization - RSC Advances (RSC Publishing) DOI:10.1039/C4RA09153A

Proposal of a mechanism for the ester formation catalyzed by SnCl2/MxOy... | Download Scientific Diagram

A DFT study of reduction of nitrobenzene to aniline with SnCl2 and hydrochloric acid - Yamabe - 2016 - Journal of Physical Organic Chemistry - Wiley Online Library

organic chemistry - Nitrobenzene reduction with (tin) Sn catalyst: Why is C-H bond cleavage preferred over O-H bond cleavage? - Chemistry Stack Exchange

A DFT study of reduction of nitrobenzene to aniline with SnCl2 and hydrochloric acid - Yamabe - 2016 - Journal of Physical Organic Chemistry - Wiley Online Library

organic chemistry - Synthesis of Almotritpane - mechanism for educational purpose - Chemistry Stack Exchange
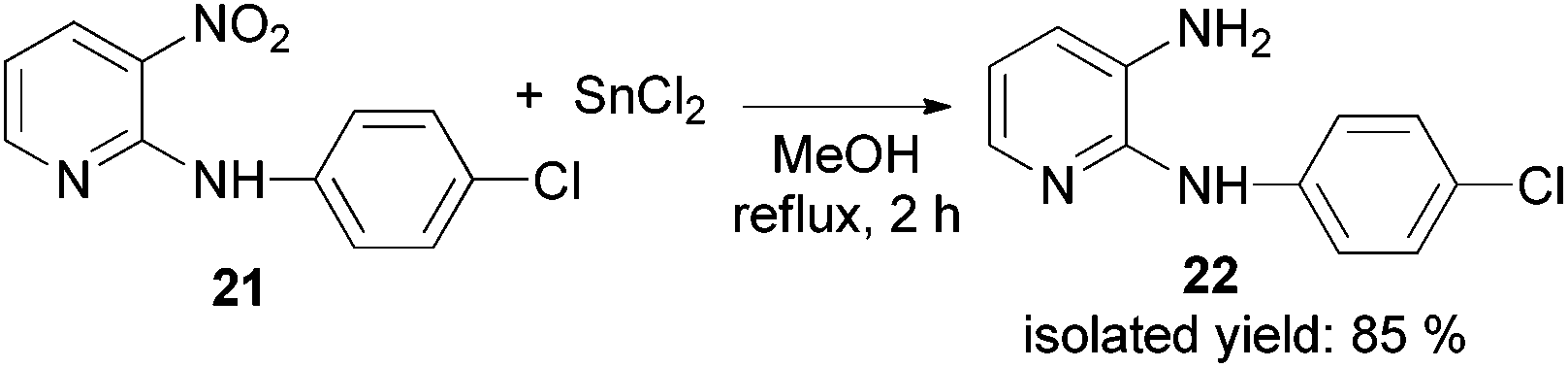
PVP-Pd nanoparticles as efficient catalyst for nitroarene reduction under mild conditions in aqueous media - Green Chemistry (RSC Publishing) DOI:10.1039/C6GC02710E

Synthesis of 2,5-disubstitued benzimidazole using SnCl2-catalyzed reduction system at room temperature - ScienceDirect

Synthesis of 2,5-disubstitued benzimidazole using SnCl<sub>2</sub>-catalyzed reduction system at room temperature





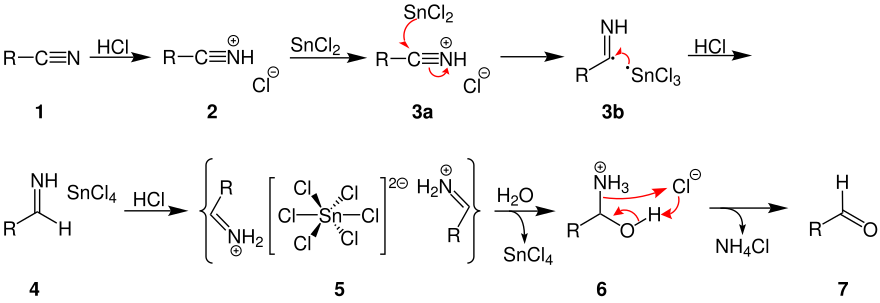


![PDF] SnCl2/TiCl3-Mediated Deoximation of Oximes in an Aqueous Solvent | Semantic Scholar PDF] SnCl2/TiCl3-Mediated Deoximation of Oximes in an Aqueous Solvent | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/f572c0db981382b43fc3efbbd7dbbe0c6b82b9e6/2-Table1-1.png)