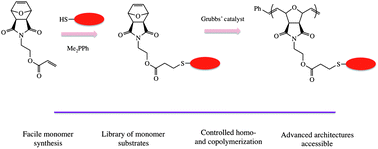
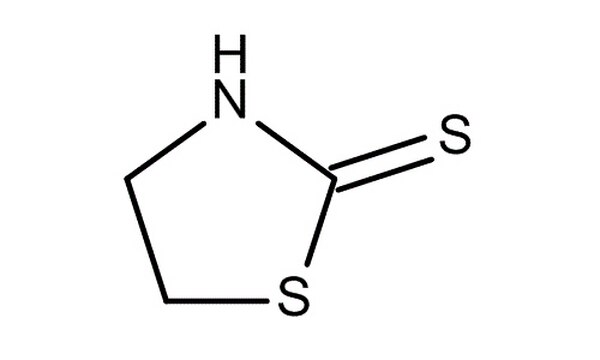
Challenging activated monomer ring-opening polymerization for direct synthesis of thiol end-functionalized polyesters - ScienceDirect
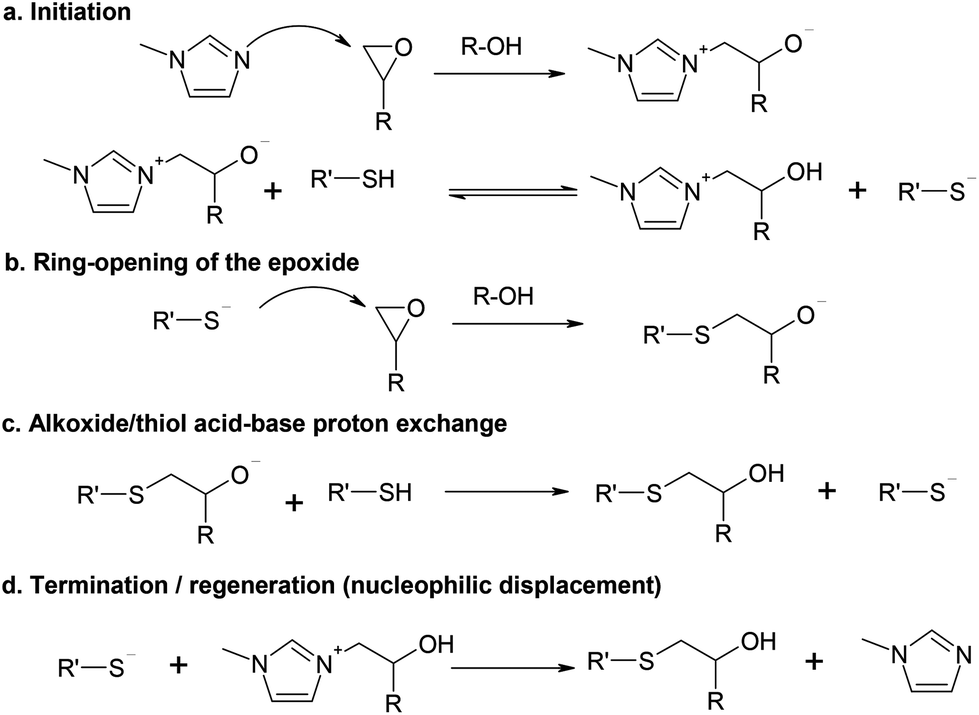
Analysis of the reaction mechanism of the thiol–epoxy addition initiated by nucleophilic tertiary amines - Polymer Chemistry (RSC Publishing) DOI:10.1039/C7PY01263B

Scheme 2 (a) The general synthetic scheme of a thiol-ene reaction. (b)... | Download Scientific Diagram

New insights in the chemical functionalization of graphene oxide by thiol-ene Michael addition reaction - ScienceDirect

Molecules | Free Full-Text | Synthetic Applications of Intramolecular Thiol-Ene “Click” Reactions | HTML

Marriage of ring-opening metathesis polymerization and thiol-maleimide chemistries: Direct polymerization of prefunctionalized monomers or postpolymerization modification? - ScienceDirect

Bis-cyclic monomer 13 with an ethylene carbonate and a thiolactone ring... | Download Scientific Diagram

Thiol-based chemical probes exhibit antiviral activity against SARS-CoV-2 via allosteric disulfide disruption in the spike glycoprotein | PNAS

Stable and Rapid Thiol Bioconjugation by Light‐Triggered Thiomaleimide Ring Hydrolysis - Kalia - 2017 - Angewandte Chemie - Wiley Online Library

Reversible ring-opening addition−fragmentation reaction of 1 in the... | Download Scientific Diagram

SciELO - Brasil - PhSeBr-catalyzed selective addition of thiols to α,β-unsaturated carbonyl compounds: regioselective synthesis of thioacetals vs. β-mercapto ketones PhSeBr-catalyzed selective addition of thiols to α,β-unsaturated carbonyl compounds ...
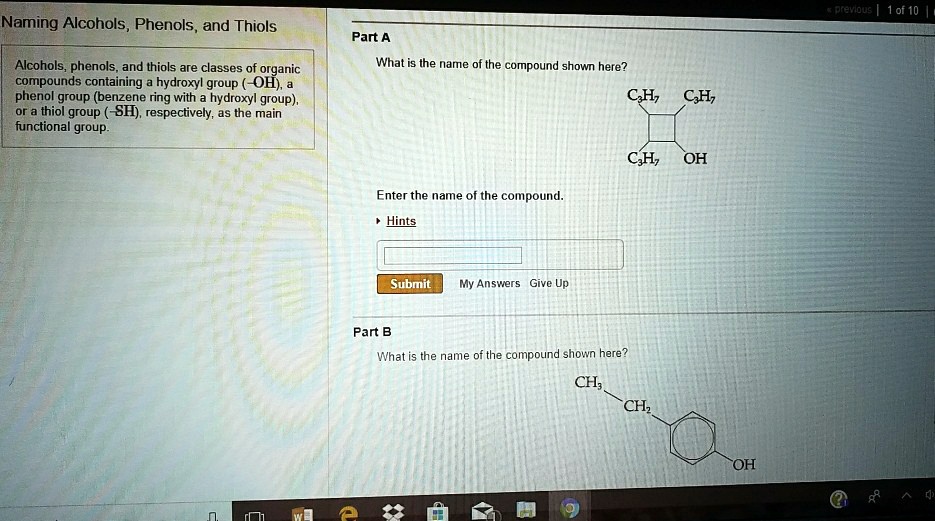
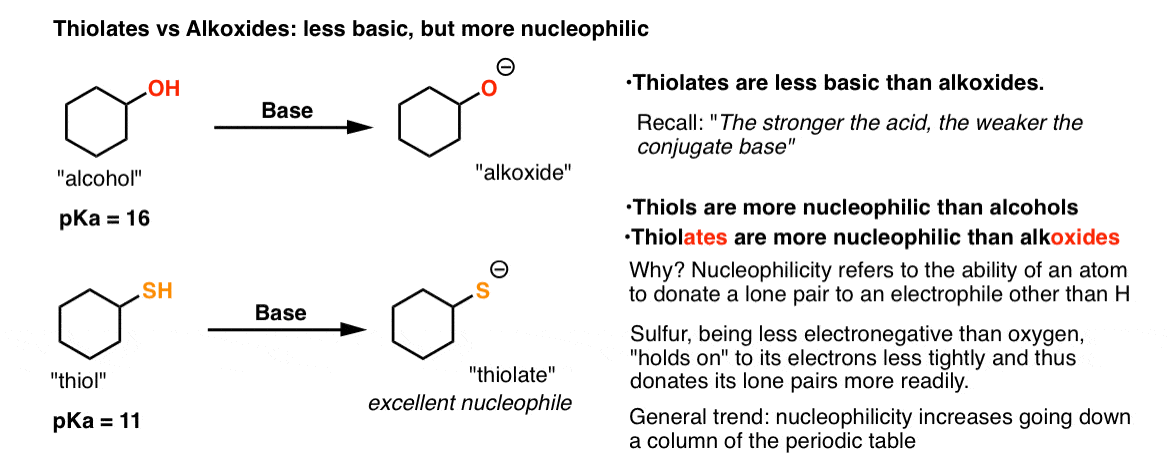
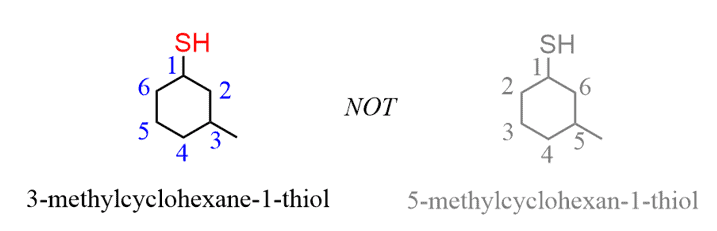
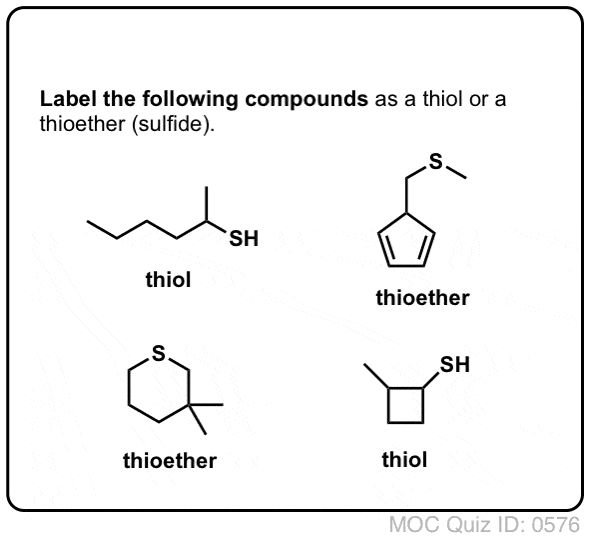
SOLVED:pretqus 1 of 10 Naming Alcohols, Phenols, and Thiols Pant A Alcohols, phenols: and thiols are classes of organic compounds containing hydroxyl group (-OH), phenol group (benzene ring - with hydroxyl group),

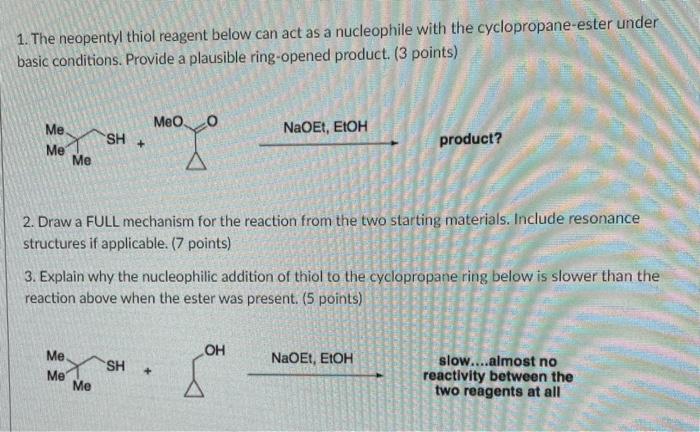
Asymmetric Ring‐Opening of Cyclopropyl Ketones with Thiol, Alcohol, and Carboxylic Acid Nucleophiles Catalyzed by a Chiral N,N′‐Dioxide–Scandium(III) Complex - Xia - 2015 - Angewandte Chemie International Edition - Wiley Online Library

Functionalized Polyphosphoester via Living Ring-opening Polymerization and Photochemical Thiol-ene Click Reaction | Semantic Scholar
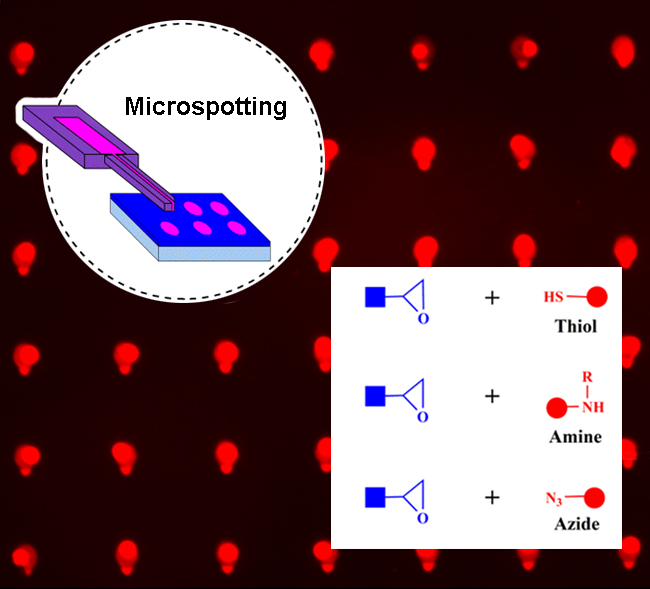
INT- Publications - 2021 - Protein Microarray Immobilization via Epoxide Ring‐Opening by Thiol, Amine, and Azide
A chemical structure of thiol-modifying reagents and their reaction... | Download Scientific Diagram