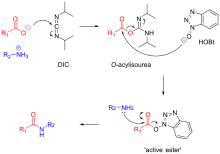
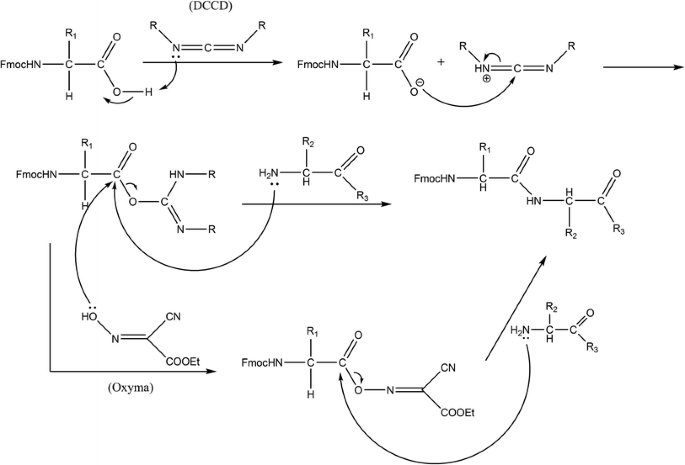
WO2014033466A1 - Method and compositions for removing acid-labile protecting groups - Google Patents

The synthesis and reactivity of optically pure amino acids bearing side-chain thioamides - Journal of the Chemical Society, Perkin Transactions 1 (RSC Publishing) DOI:10.1039/B004688O

1,4-Benzenedimethanethiol (1,4-BDMT) as a scavenger for greener peptide resin cleavages - RSC Advances (RSC Publishing)

An important side reaction using the thiol, 3,6‐dioxa‐1,8‐octanedithiol (DODT), in 9‐fluorenylmethoxycarbonyl‐based solid phase peptide synthesis - Harris - 2014 - Journal of Peptide Science - Wiley Online Library
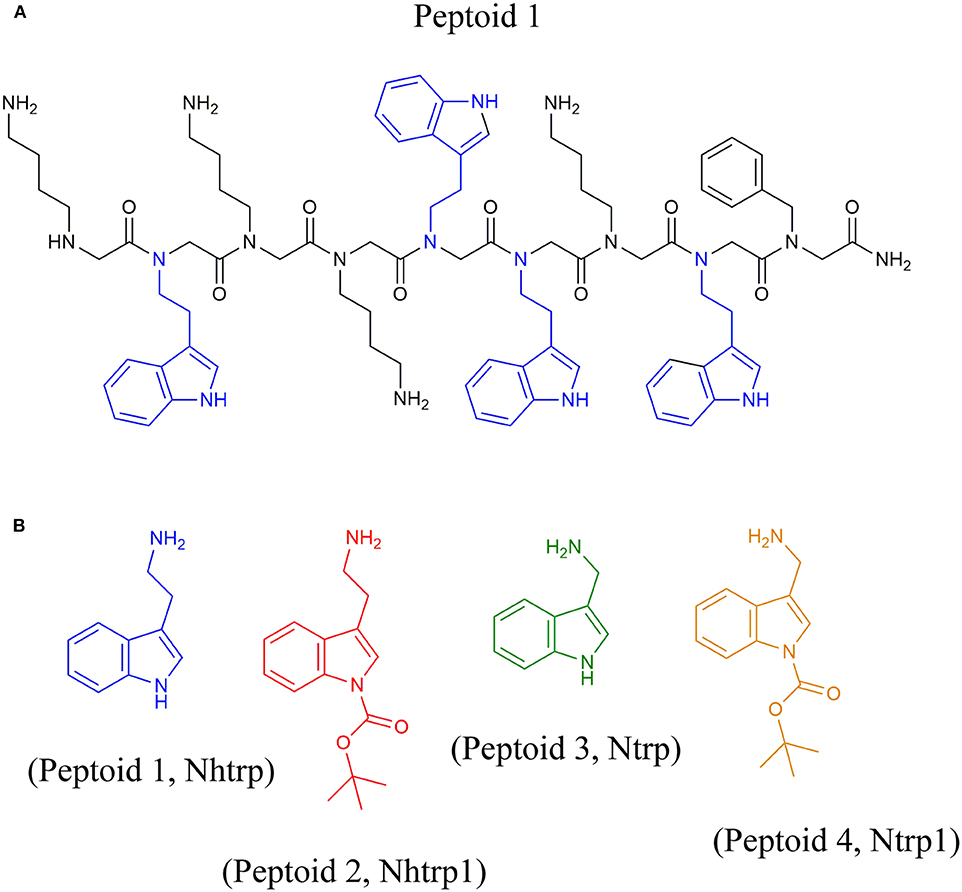
Frontiers | Synthesis of Peptoids Containing Multiple Nhtrp and Ntrp Residues: A Comparative Study of Resin, Cleavage Conditions and Submonomer Protection | Chemistry

The renascence of continuous-flow peptide synthesis – an abridged account of solid and solution-based approaches - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/C7OB02759A
An important side reaction using the thiol, 3,6â•'dioxaâ•'1,8â•'octanedithiol (DODT), in 9â•'fluorenylmethox