LiCl-mediated, easy, and low-cost removal of the trityl group from protected alcohols and diols - ScienceDirect

SciELO - Brasil - Solid-Phase Peptide Synthesis of Dipeptide (Histidine-β-Alanine) as a Chelating Agent by Using Trityl Chloride Resin, for Removal of Al<sup>3+</sup>, Cu<sup>2+</sup>, Hg<sup>2+</sup> and Pb<sup>2+</sup>: Experimental and Theoretical ...
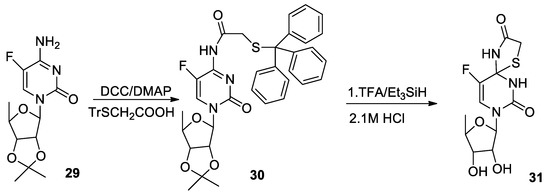
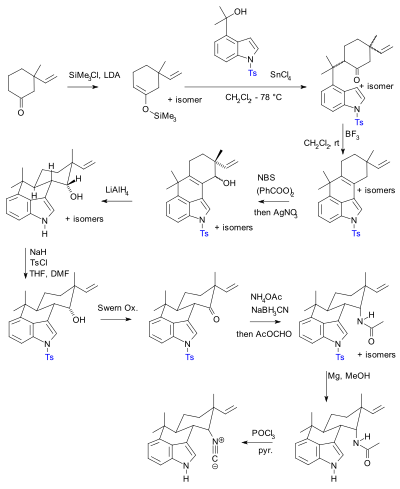
Molecules | Free Full-Text | Synthesis of Thiol Derivatives of Biological Active Compounds for Nanotechnology Application | HTML
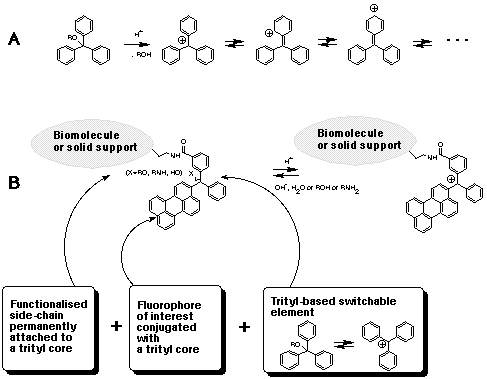
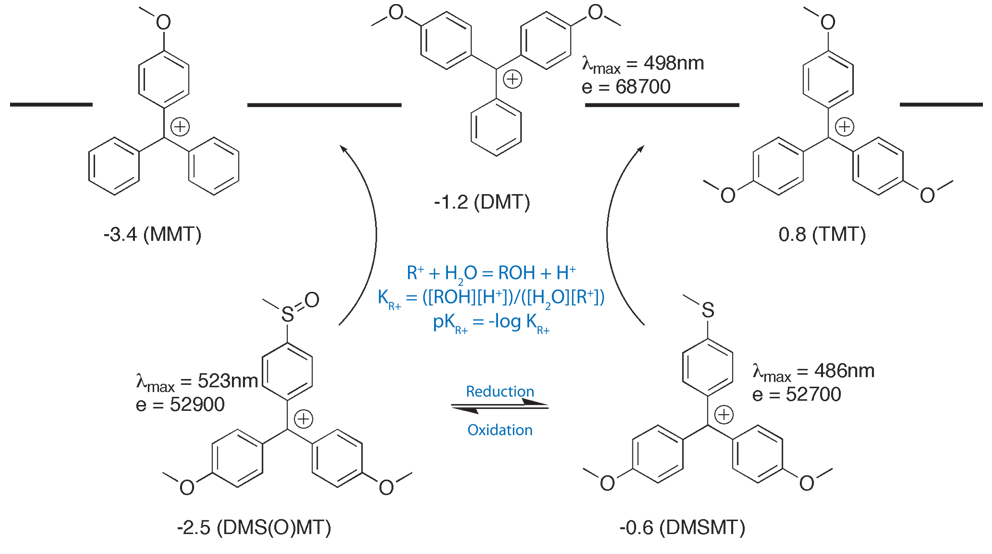
Glen Report 13.13 - Trityl Group in the 3rd Millenium:<br> New Perspectives for Oligonucleotide Chemistry and Beyond

Synthesis of Peptides Containing C-Terminal Esters Using Trityl Side-Chain Anchoring: Applications to the Synthesis of C-Terminal Ester Analogs of the Saccharomyces cerevisiae Mating Pheromone a-Factor. - Abstract - Europe PMC
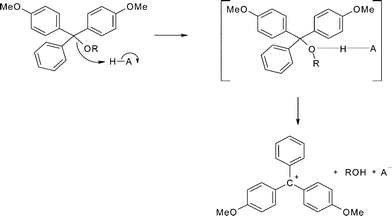
The kinetics and mechanism of the acid-catalysed detritylation of nucleotides in non-aqueous solution - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/B816235B

Prevention of aspartimide formation during peptide synthesis using cyanosulfurylides as carboxylic acid-protecting groups | Nature Communications
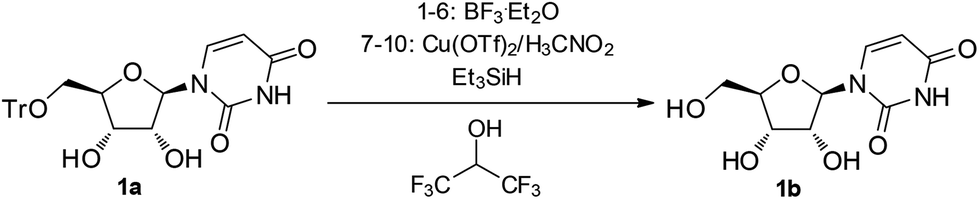
A three-component reagent system for rapid and mild removal of O -, N - and S -trityl protecting groups - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/C6OB00067C
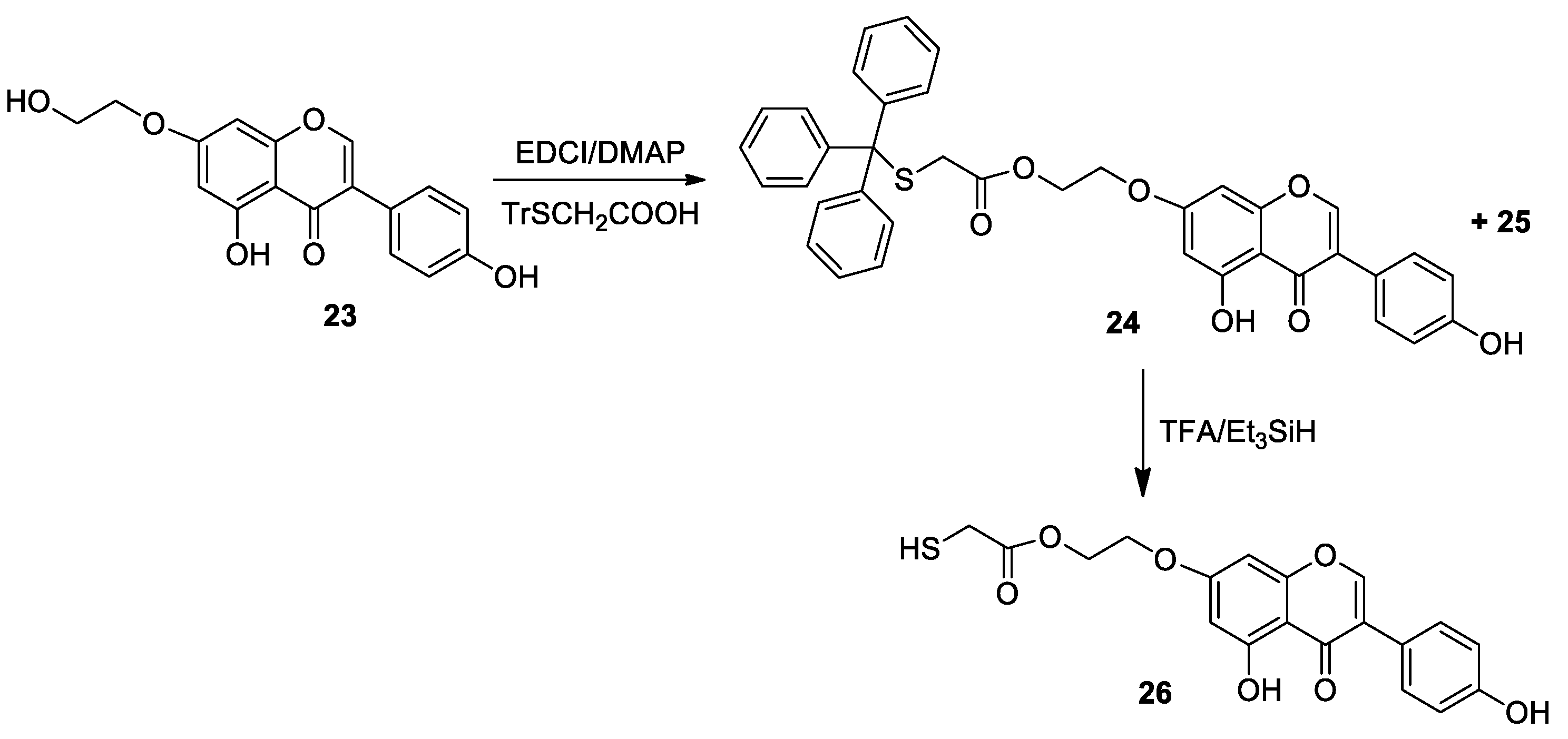
Molecules | Free Full-Text | Synthesis of Thiol Derivatives of Biological Active Compounds for Nanotechnology Application | HTML
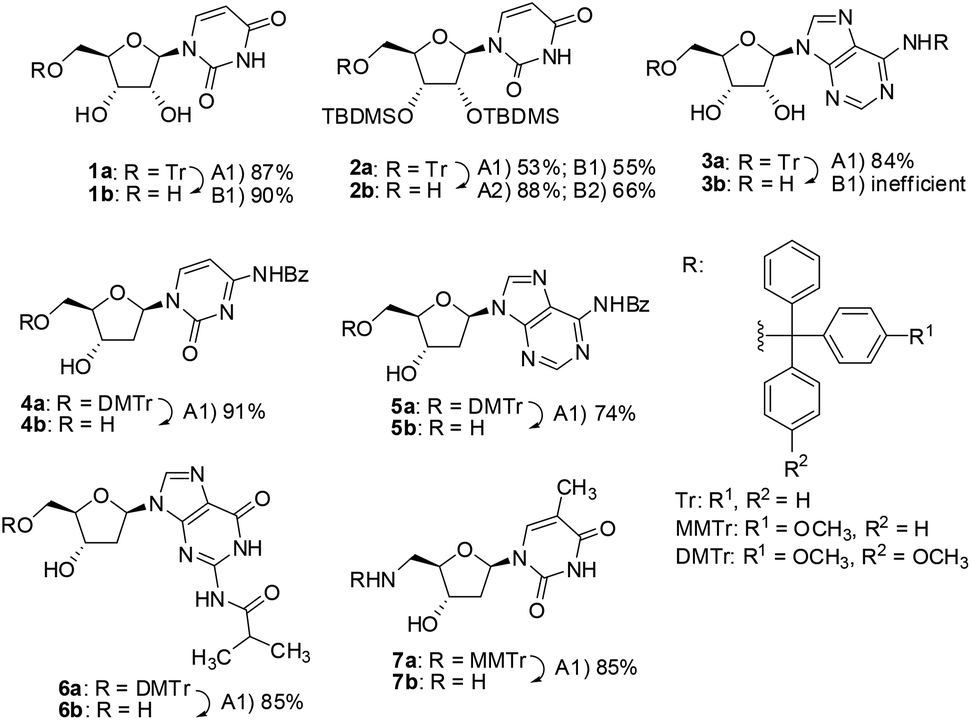
A three-component reagent system for rapid and mild removal of O -, N - and S -trityl protecting groups - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/C6OB00067C
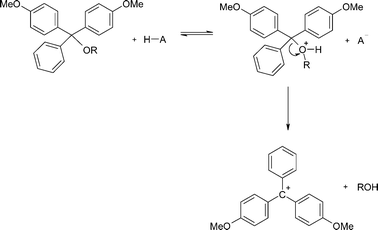
The kinetics and mechanism of the acid-catalysed detritylation of nucleotides in non-aqueous solution - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/B816235B

Stabilities of Trityl‐Protected Substrates: The Wide Mechanistic Spectrum of Trityl Ester Hydrolyses - Horn - 2010 - Chemistry – A European Journal - Wiley Online Library
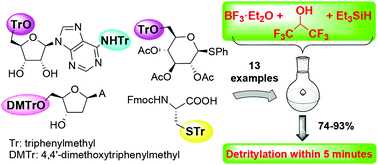
A three-component reagent system for rapid and mild removal of O-, N- and S- trityl protecting groups - Organic & Biomolecular Chemistry (RSC Publishing)